Laninamivir octanoate preparation method
A technology of miviroctate and la nina, which is applied in the field of neuraminic acid derivatives, can solve the problems of increased use of organic solvents, unsuitable for industrial production, and difficulty in purchasing raw materials, and achieves simple equipment, easy quality control, and ease of use. The effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
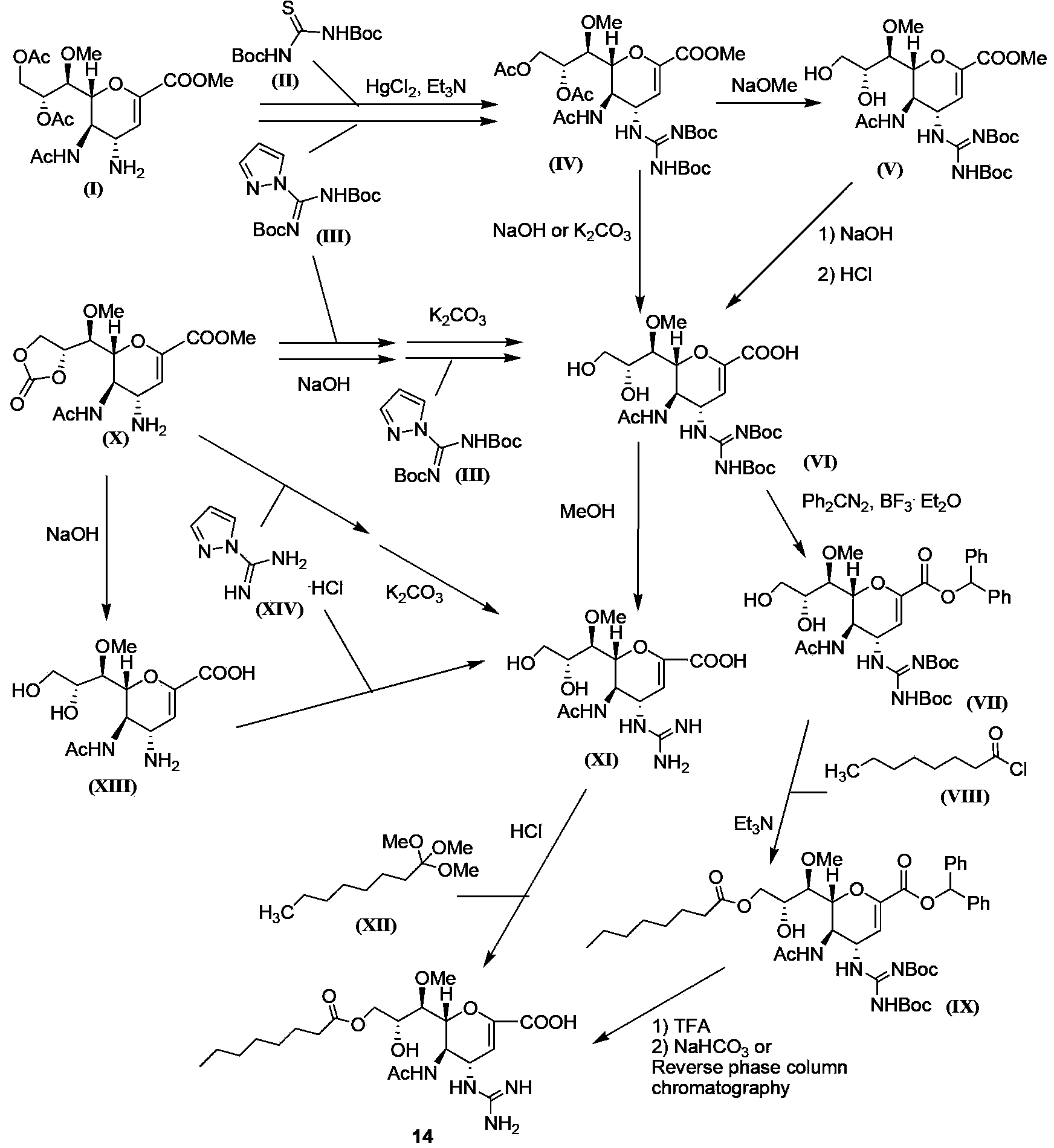
Image
Examples
Embodiment 1
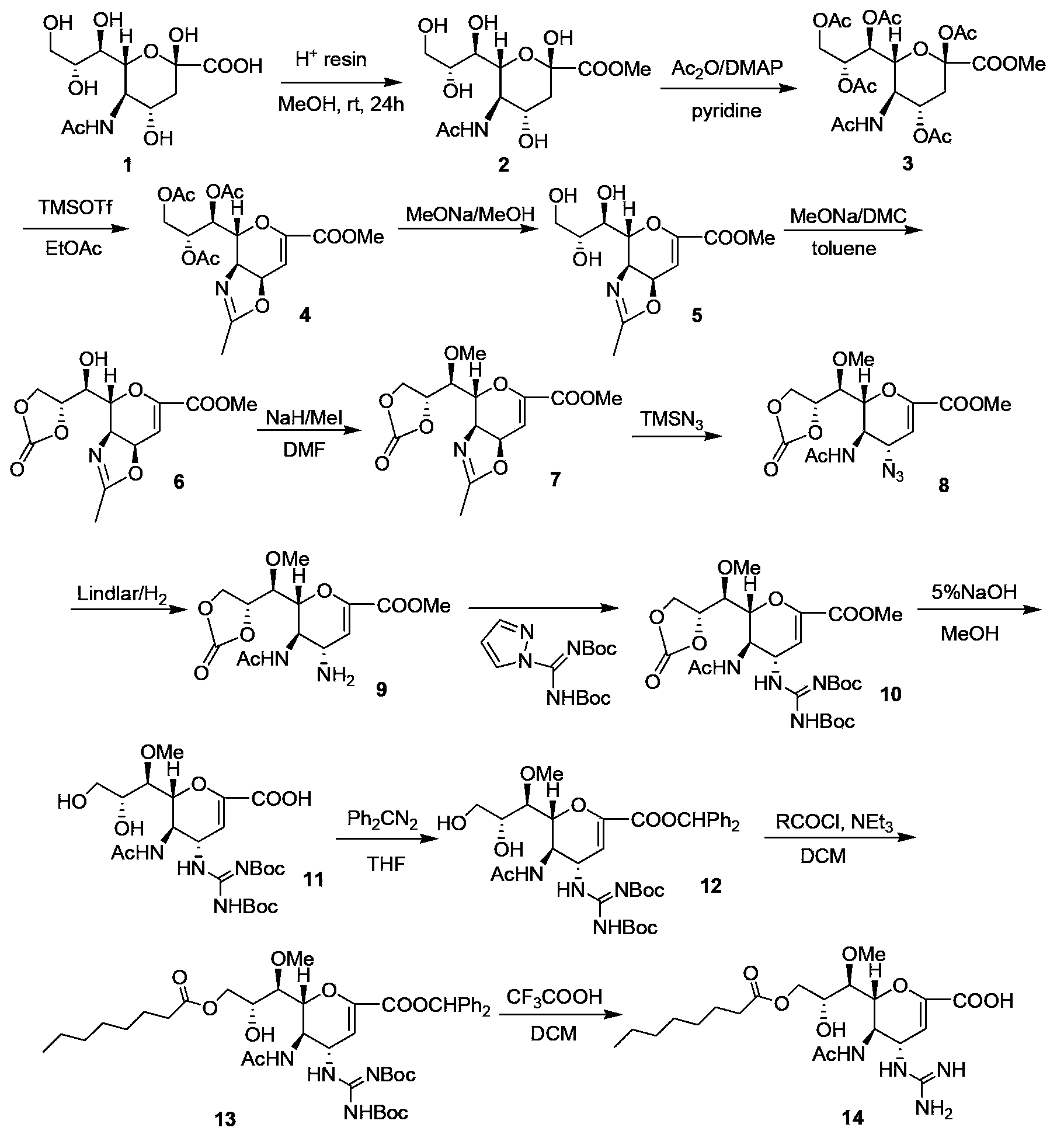
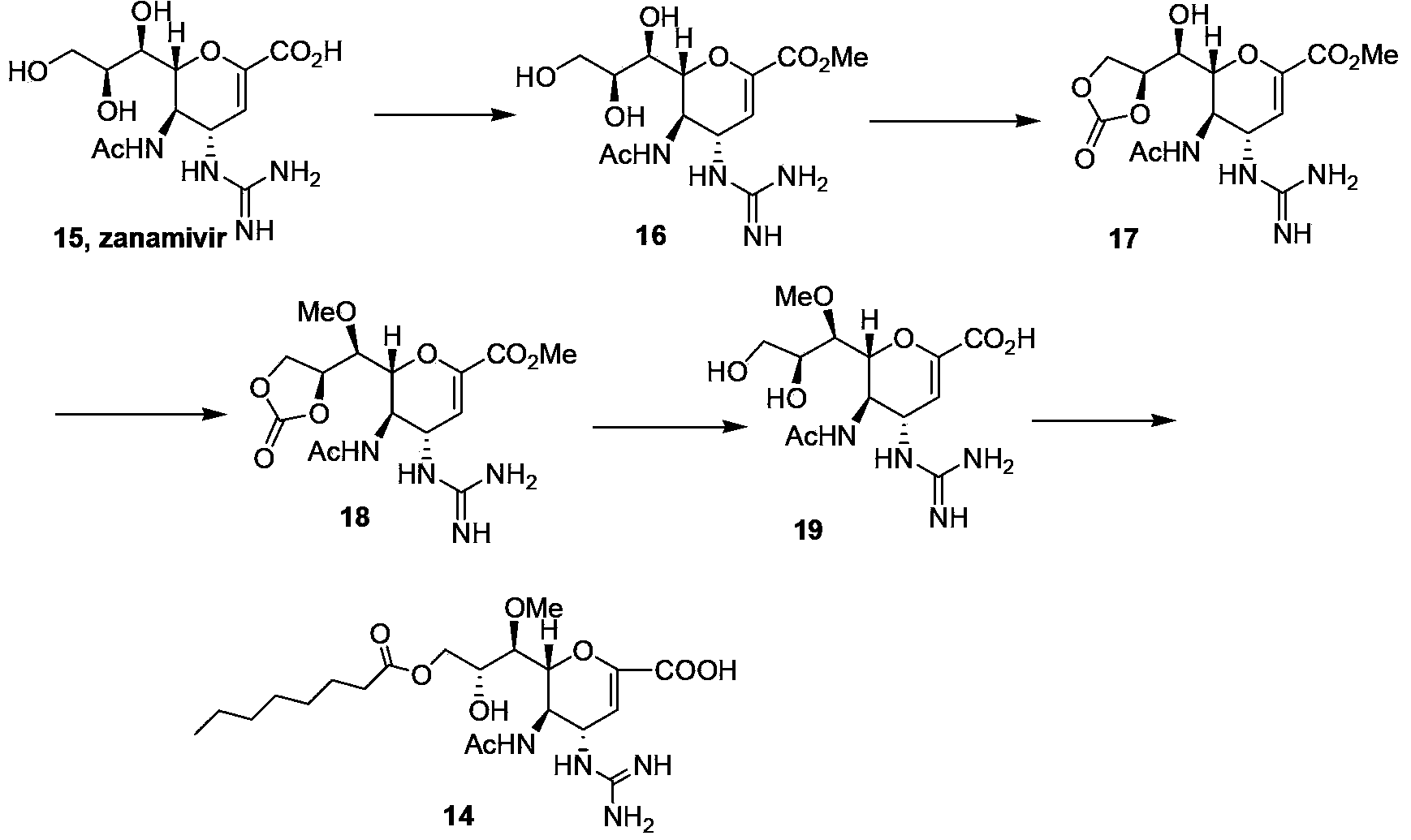
[0034] The preparation of embodiment 1 laninamivir axetil
[0035] Synthesis of formula 16 compound zanamivir methyl ester
[0036] In a 20L reactor, a suspension was prepared by mixing zanamivir (109.7g, 0.33mol) with 6.9L of methanol. Add an acidic positive resin selected from Dowex50 (H+) (55g) to the suspension, and react at room temperature for 30 hours; complete, remove the resin by filtration, wash with methanol (0.1L×2), and concentrate the filtrate to obtain an off-white solid, namely compound 16 , yield 98.1%.
[0037] Synthesis of compounds of formula 17
[0038] Mix the compound of formula 16 (103.8g, 0.30mol) and toluene (300ml) at room temperature, then add dimethyl carbonate (63.2ml) and methanol solution of sodium methoxide (0.3ml) respectively, and heat the mixture to 80°C, Reflux reaction for 5 hours; after the reaction is completed, cool down to 0°C, stir for 15 minutes, let stand to crystallize, filter, and wash with toluene (50ml×2) to obtain a light ye...
Embodiment 2
[0045] The preparation of embodiment 2 laninamivir axetil
[0046] Synthesis of the compound of formula 16
[0047] In a 20L reactor, a suspension was prepared by mixing zanamivir (36.6g, 0.11mol) with 0.92L methanol. Add an acidic positive resin selected from Bio-Rad (H+) (11.0g) to the suspension, and react at room temperature for 10 hours; complete, remove the resin by filtration, wash with methanol (30mL×2), and concentrate the filtrate to obtain an off-white solid, namely Formula 16 compound, yield 90.6%.
[0048] Synthesis of compounds of formula 17
[0049] The compound of formula 16 (34.6g, 0.10mol) was mixed with tetrahydrofuran (100ml) at room temperature, then dimethyl carbonate (9.3ml) and methanol solution of sodium methoxide (0.06ml) were added respectively, and the mixture was heated to 50°C, Reflux reaction for 4 hours; after the reaction was completed, cool down to 0°C, stir for 20 minutes, stand for crystallization, filter, wash with toluene (15ml×2), and ...
Embodiment 3
[0056] The preparation of embodiment 3 laninamivir axetil
[0057] Synthesis of the compound of formula 16
[0058] In a 20L reactor, a suspension was prepared by mixing zanamivir (54.8g, 0.16mol) with 6.94L of methanol. Add an acidic positive resin selected from Dowex50 (H+) (54.8g) to the suspension, and react at 70°C for 72 hours; after completion, remove the resin by filtration, wash with methanol (50mL×2), and concentrate the filtrate to obtain an off-white solid, the compound Formula 16 compound, yield 94.2%.
[0059] Synthesis of compounds of formula 17
[0060] Mix the compound of formula 16 (51.9g, 0.15mol) and 1,4-dioxane (150ml) at room temperature, then add dimethyl carbonate (63.2ml) and methanol solution of sodium methoxide (0.8ml) respectively. The mixed solution was heated to 100°C, and refluxed for 8 hours; after the reaction was completed, the temperature was lowered to 0°C, stirred for 15 minutes, left to stand for crystallization, filtered, washed with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com