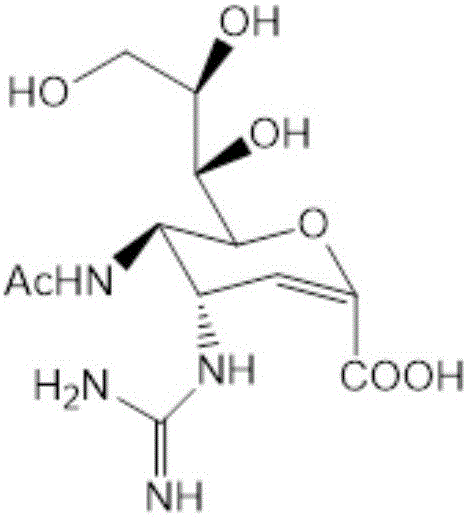
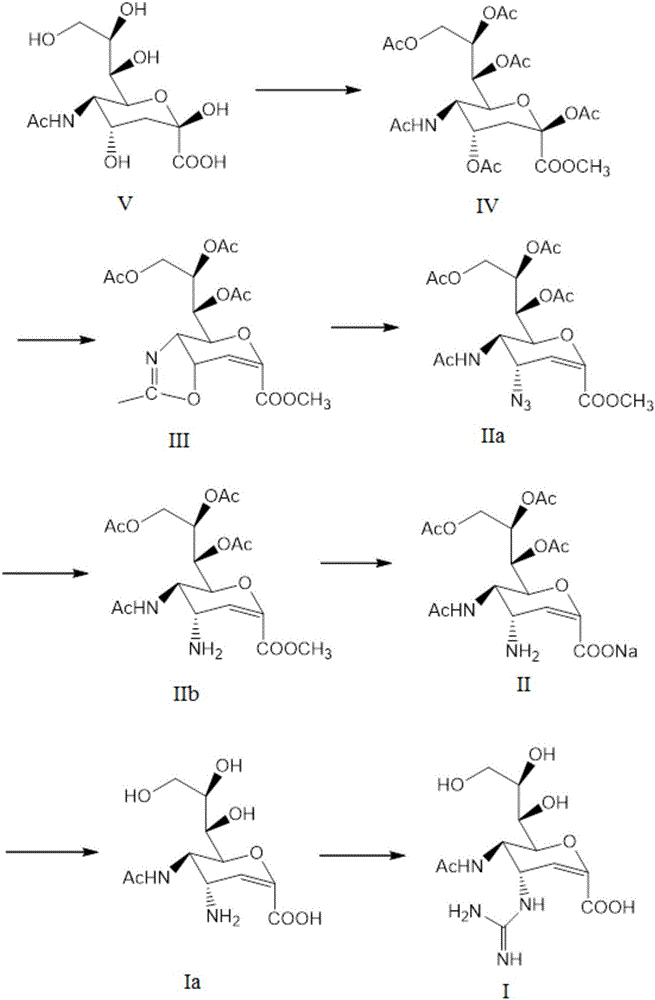
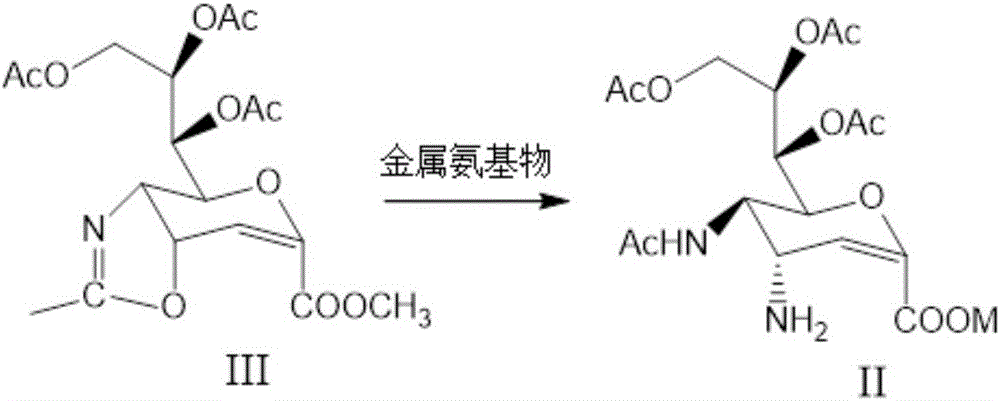
Preparation method of zanamivir intermediate and preparation method of zanamivir
A technology of zanamivir and body acetyl, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high price, high price, and difficult industrialization of silicon trimethyl azide, and achieve the goal of being suitable for large-scale industrialization Production, reduction of production cost, and the effect of shortening the synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Synthesis of acetylated protected amino compounds (formula II)
[0094] To 120 g of cyclic compound (formula III), add 1200 g of anhydrous tetrahydrofuran, and slowly add 20 g of potassium amide. Stir, heat, and react at the reflux temperature of tetrahydrofuran for 7 hours. 80 g of water was added to quench the reaction. Concentrate, add ethyl acetate to dissolve, wash with 300g water three times each time, concentrate ethyl acetate to dryness. Recrystallize with 550g of isopropanol, filter and dry to obtain 123g of acetylated amino compound (Formula II). Yield: 93.1%.
Embodiment 2
[0096] (1) Synthetic sialic acid (formula IV) with protective group
[0097]Sialic acid (formula V) 100g, methanol 1000g, strong acid ion exchange resin 5g; stirring at room temperature for 12 hours, the reaction solution was a dissolved and clear solution. As detected by HPLC, the normalized content of sialic acid was 1.2%. After filtration, the filtrate was concentrated in methanol to dryness to obtain methyl sialic acid.
[0098] 1000 g of pyridine and 3 g of p-dimethylaminopyridine (DMAP) were added, and 250 g of acetic anhydride was added under ice-water cooling. Stir at room temperature for 15 hours. As detected by HPLC, the normalized content of methyl sialic acid was 0.3%. Pyridine was concentrated to dryness. Dissolve with 1000g ethyl acetate, wash with 300g water three times each time, and concentrate ethyl acetate to dryness. 169 g of sialic acid (formula IV) with protective groups was obtained. Yield: 97.9%.
[0099] (2) Synthetic ring compound (formula III)...
Embodiment 3
[0109] (1) Synthetic sialic acid (formula IV) with protective group
[0110] In a 30L glass reactor, add 2 kg of sialic acid (formula V), 20 kg of methanol, and 100 g of strong acid ion exchange resin; stir at room temperature for 10 hours, and the reaction solution becomes a dissolved and clear solution. HPLC detection, sialic acid normalization method content is 1.0%. After filtration, the filtrate was transferred to a 30L glass reactor, and the methanol was concentrated to dryness to obtain methyl sialic acid.
[0111] Add 20kg of pyridine, 80g of p-dimethylaminopyridine (DMAP), and add 5kg of acetic anhydride under ice-water cooling. Stir at room temperature for 15 hours. As detected by HPLC, the normalized content of methyl sialic acid was 0.3%. Pyridine was concentrated to dryness. Add 20kg of ethyl acetate to dissolve, wash with 6L of water three times each time, and concentrate the ethyl acetate to dryness. 3.4 kg of sialic acid (formula IV) with protective groups...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com