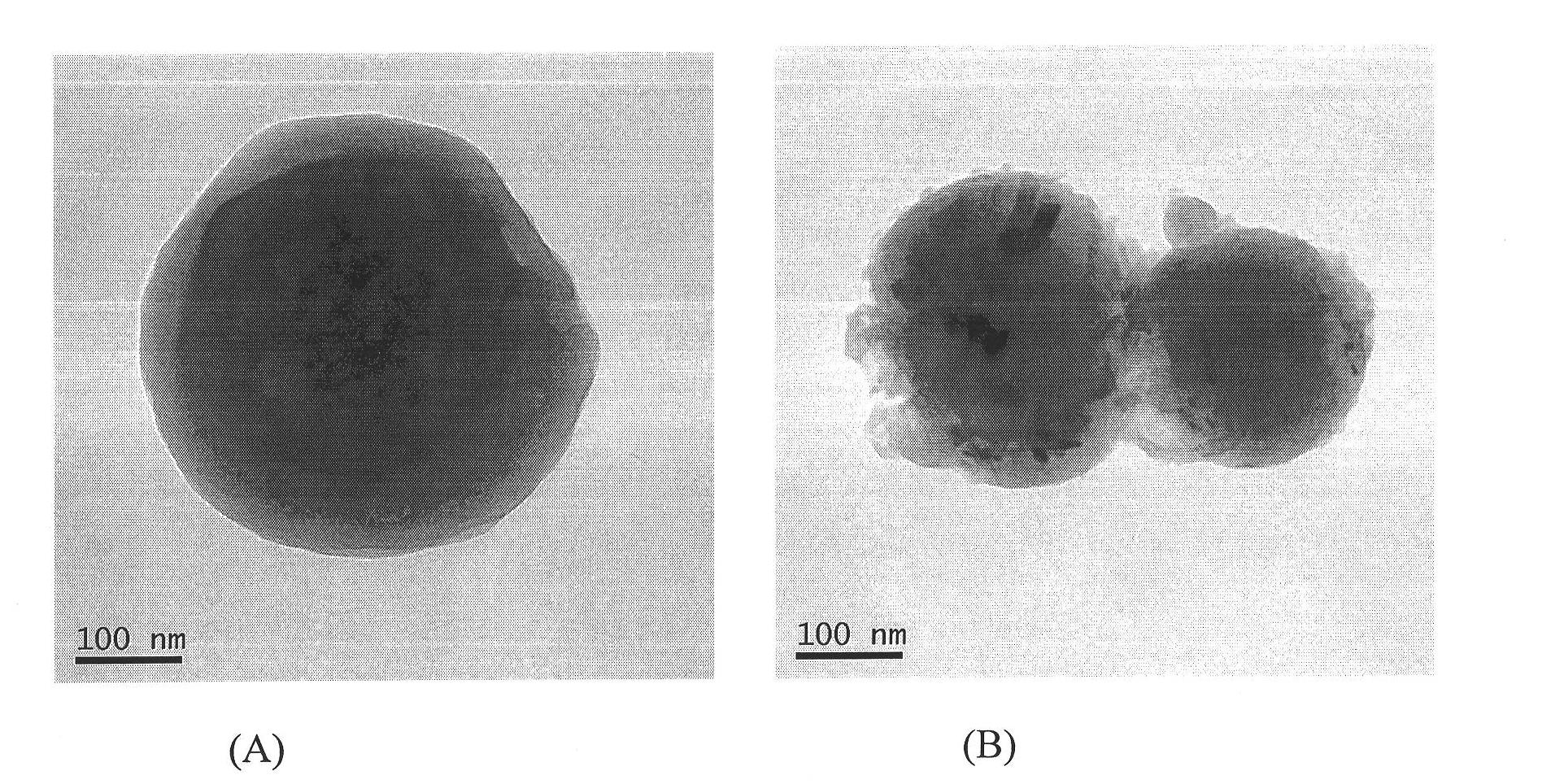
Zanamivir solid lipid nanosphere oral preparation and preparation method thereof
A kind of solid lipid nanometer and lipid material technology, applied in the field of new pharmaceutical dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of blank nanoparticles
[0021] Take 0.2ml of distilled water (inner water phase) and add it to 1ml of the oil phase. After being dispersed by a disperser, put it in an ultrasonic cell pulverizer for 200w and 60s to form W / O colostrum. Then add colostrum into 4ml of Proxamer 188 solution (1.6% w / v, external water phase), after being dispersed by a disperser, put it in an ultrasonic cell pulverizer at 200w, 30s and form W / O / W Double milk. The prepared double emulsion was placed on a magnetic stirrer at 25° C., 700 rpm, and stirred for 5 hours to evaporate the organic solvent to obtain a nanosuspension. For the oil phase, 120 mg of glyceryl monostearate and 40 mg of soybean lecithin were melted in 1 ml of dichloromethane, and mixed thoroughly to obtain a uniform oil phase. Add 5% mannitol to the nanosuspension as a freeze-drying protective agent, set the shelf temperature to -30°C, and vacuum degree to 0.1mbar. After pre-freezing in a -70°C ref...
Embodiment 2
[0022] Embodiment 2: Preparation of blank nanoparticles
[0023] Take 0.2ml of distilled water (inner water phase) and add it to 1ml of the oil phase. After being dispersed by a disperser, put it in an ultrasonic cell pulverizer for 200w and 60s to form W / O colostrum. Then add colostrum into 8ml of Proxamer 188 solution (1.6% w / v, external water phase), after being dispersed by a disperser, put it in an ultrasonic cell pulverizer at 200w and 30s to form W / O / W Double milk. The prepared double emulsion was placed on a magnetic stirrer at 25° C., 700 rpm, and stirred for 5 hours to evaporate the organic solvent to obtain a nanosuspension. For the oil phase, 120 mg of glyceryl monostearate and 40 mg of soybean lecithin were melted in 1 ml of dichloromethane, and mixed thoroughly to obtain a uniform oil phase.
Embodiment 3
[0024] Embodiment 3: Preparation of blank nanoparticles
[0025]Take 0.2ml of distilled water (inner water phase) and add it to 1ml of the oil phase. After being dispersed by a disperser, put it in an ultrasonic cell pulverizer for 200w and 60s to form W / O colostrum. Then add colostrum into 12ml of Proxamer 188 solution (1.6% w / v, external water phase), after being dispersed by a disperser, put it in an ultrasonic cell pulverizer at 200w, 30s and form W / O / W Double milk. The prepared double emulsion was placed on a magnetic stirrer at 25° C., 700 rpm, and stirred for 5 hours to evaporate the organic solvent to obtain a nanosuspension. For the oil phase, 120 mg of glyceryl monostearate and 40 mg of soybean lecithin were melted in 1 ml of dichloromethane, and mixed thoroughly to obtain a uniform oil phase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com