Zanamivir inhalation solution and application thereof
A zanamivir, solution technology, applied in the field of zanamivir inhalation solution, to achieve the effect of increasing deposition rate, large curative effect, and reducing absorption difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of zanamivir inhalation solution
[0043]
[0044] The preparation process in Example 1.1 is as follows: Accurately weigh quantitative glucose and zanamivir, add to 900g of water, stir with a mechanical stirrer (IKA RW20 digital) at 150 rpm until completely dissolved, and finally add the remaining water to 1000g
[0045] Embodiment 1.2 and embodiment 1.3 are prepared by the same process: accurately weigh the quantitative surfactant and osmotic pressure regulator, add it to 800g of water, put the liquid into a shaker, and shake it at a frequency of 45 times per minute to completely dissolved. Then add quantitative zanamivir raw material, and continue to shake at a frequency of 60 times per minute until completely dissolved. Finally the remaining water was added to make up to 1000 g.
[0046] Example 1.4 and Example 1.5 were prepared using the same process: accurately weigh the quantitative surfactant and osmotic pressure regulator, add it to 800g of wate...
Embodiment 2
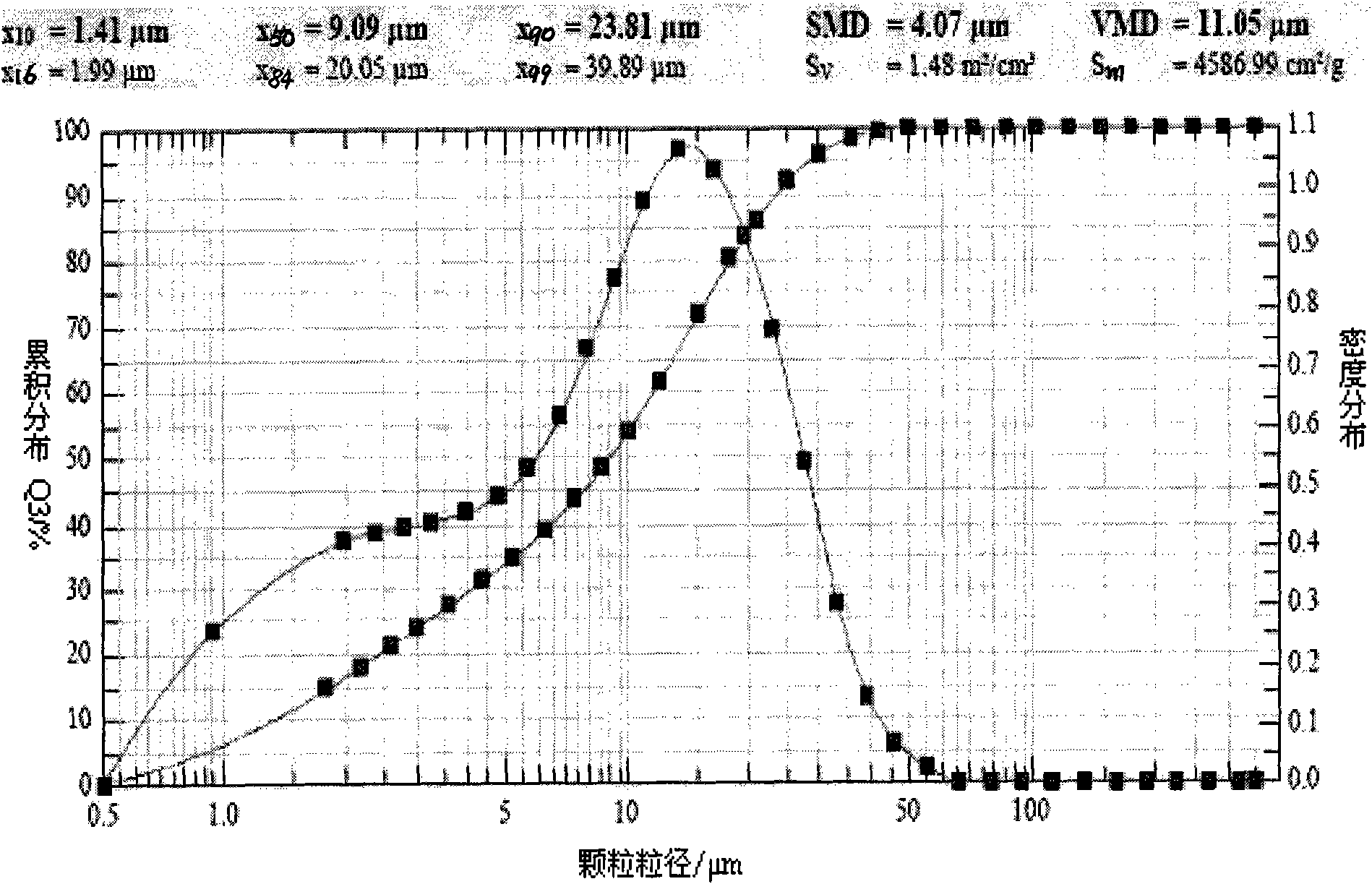
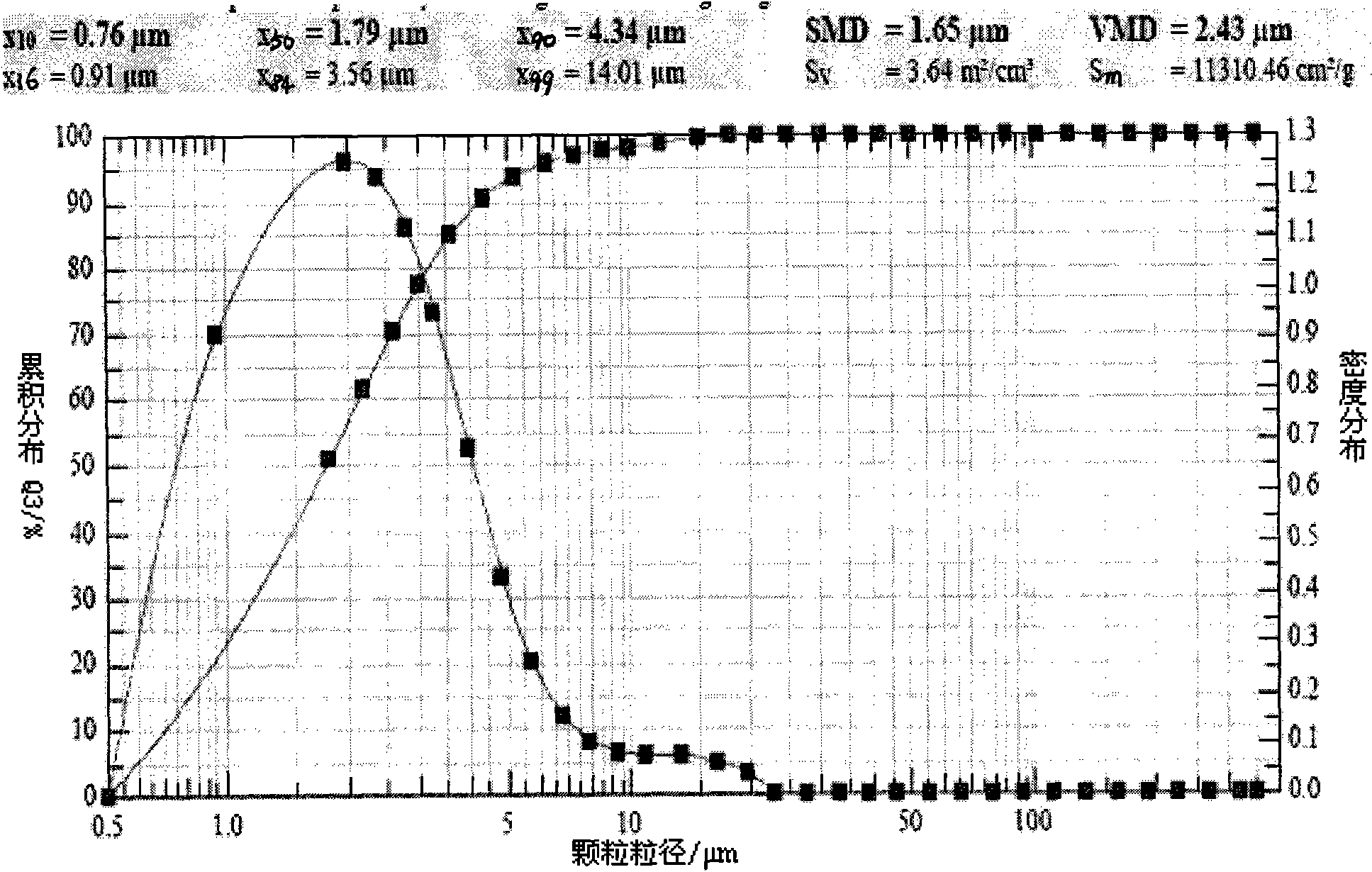
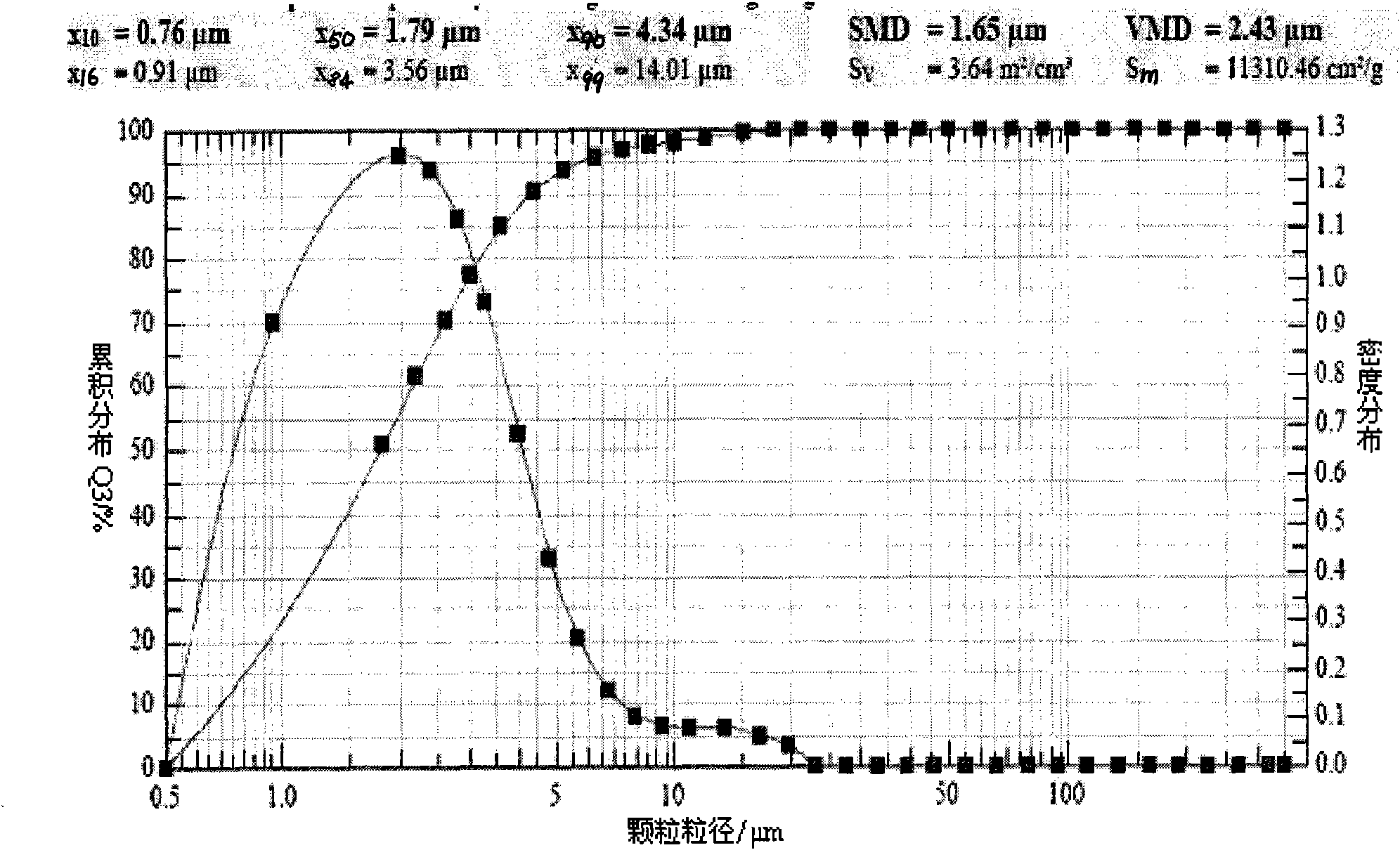
[0049] Detect the ultrasonic atomization inhalation device according to the operating instructions, install the pipeline, add 90ml of cold distilled water in the water tank, and then add 10ml of the zanamivir solution of Example 1.1. Make sure the liquid level is submerged in the sound-permeable membrane at the bottom of the tank. Tighten the lid of the can, place the atomizer in the sink, and close the lid of the sink. Power on, preheating: power on and preheat for 3 minutes. Adjust the ultrasonic frequency to be 1.5MHz, and the atomization speed is 2ml / min. The atomization nozzle is connected with a micron laser particle size analyzer (SympatecHELOS, Germany), and the volume average particle diameter ( figure 1 ); Get embodiment 1.4 and 1.6 to operate in the same way, measure its volume average particle diameter respectively ( figure 2 , image 3 ). The volume average particle diameter VMD of embodiment 1.1 is 11.05 μ tm, X 90 It is 23.81 μm; the volume average particl...
Embodiment 3
[0051] Detect the ultrasonic atomization inhalation device according to the operating instructions, install the pipeline, add 90ml of cold distilled water in the water tank, and then add 10ml of the zanamivir inhalation solution of Example 1.4. Make sure the liquid level is submerged in the sound-permeable membrane at the bottom of the tank. Tighten the lid of the can, place the atomizer in the sink, and close the lid of the sink. Power on, preheating: power on and preheat for 3 minutes. Adjust the ultrasonic frequency to 1.5MHz, and the atomization speed is 2ml / min, connect the atomization nozzle to the micron-scale laser particle size analyzer (Sympatec HELOS, Germany), and the atomization nozzle is 20cm away from the laser particle size analyzer to measure the inlet. The nozzles are connected with hoses of the same diameter. Measure the volume average particle diameter ( Figure 4 ); Get embodiment 1.6 and operate in the same way, measure the volume average particle diam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com