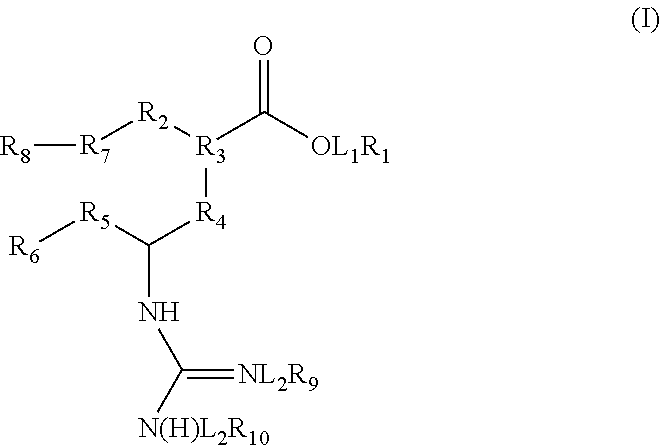
Prodrugs of Neuraminidase Inhibitors
a technology of neuronalinoleic acid and prodrugs, which is applied in the direction of drug compositions, peptides, group 4/14 element organic compounds, etc., can solve the problems of unpredictable transport and release of active agents from transport species, poor bio-pharmaceutical properties of many potentially effective therapeutic agents, and less desirable drug candidates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0060]Parent compounds, such as zanamivir can be purchased commercially or synthesized.
[0061]The synthesis of zanamivir is shown in Scheme 1. The starting material used for zanamivir synthesis is sialic acid 1, which was converted to the methyl ester 2, in presence of Dowex H+ as described in detail in reference 104. The hydroxyl groups of 2 are protected with acetyl groups to give compound 3, which was then converted to the oxazoline derivative 4 in the presence of trimethyltrifluoromethanesulfonate as described in detail in reference 105. Azide 5 was synthesized from 4 in presence of azidotrimethylsilane as described in detail in reference 105. The azide is reduced to the corresponding amine 6 by using Lindlar's catalyst, and the amine is in turn converted to the guanidine derivative 7 as described in detail in reference 106. The final step involves the deprotection of the methyl ester and acetyl groups in the presence of methanolic sodium hydroxide to give Boc-protected zanamivir...
example 2
Ethoxyester Derivatives of Zanamivir
[0062]Synthetic steps for ethoxyester derivatives of zanamivir are summarized in Scheme 2. Intermediate 10 was synthesized from intermediate 8 and α-chloro methylester of respective R group as described in detail in reference 107. The alkyl α-chloro methylester was synthesized from respective carbonyl chloride in the presence of zinc chloride and acetaldehyde as described in detail in reference 107. For the compounds with amino acid substitution instead of an alkyl group, the α-chloro methylester was synthesized from the cesium salt of respective Boc-protected amino acid in presence of 1-chloro-1-bromoethane as described in detail in reference 108. Compound 11 is synthesized from 10 using coupling conditions. The final step involves the deprotection of the Boc-protecting group to give final compounds 4 a-x. Zanamivir-Valine (Zan-Val), 1H NMR (CD3OD) δ (ppm) 6.9 (q, 1H), 5.8 (d, J=2.0 Hz, 1H), 5.2 (m, 1H), 4.9 (m, 1H), 4.2 (m, 2H), 4.02 (m, 2H), 3....
example 3
Amino Acid Ester Prodrugs of Zanamivir
[0063]L-valine, L-leucine, L-isoleucine and L-phenylalanine ester prodrugs of zanamivir, shown below, are synthesized. For comparison, the free carboxylic group of zanamivir is esterified with an ethyl group initially, and the effect of the higher alcohol esters on the permeability properties is determined. The synthesis is also functional in the β and γ amino acid analogs of Val, Ile, Leu and Phe with comparable yields to those detailed above. D-alanine, α-amino acid, D-methionine α-amino acid and L-phenyl glycine are also prepared in good yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com