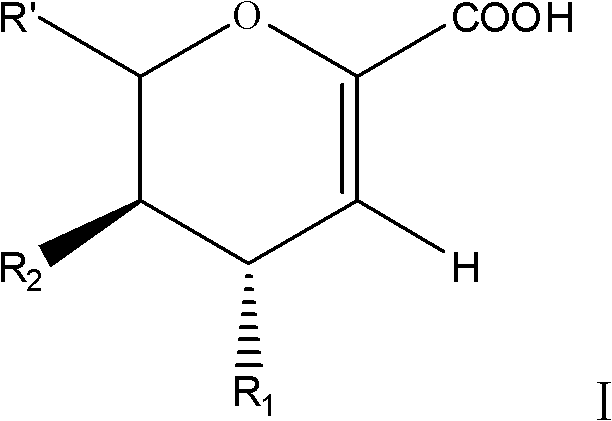
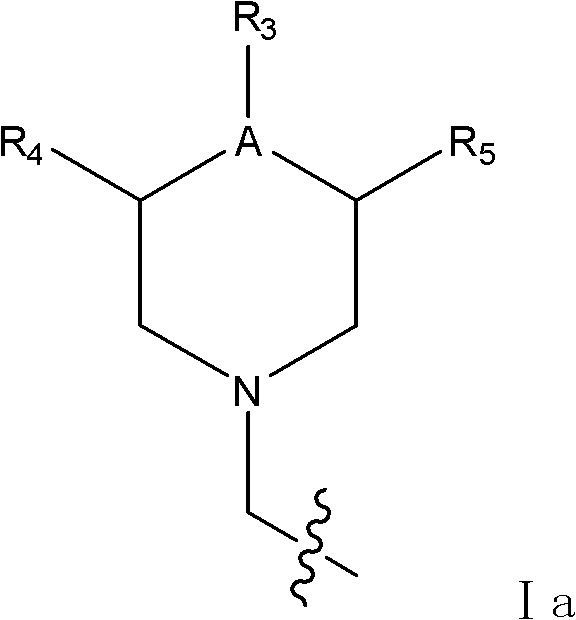
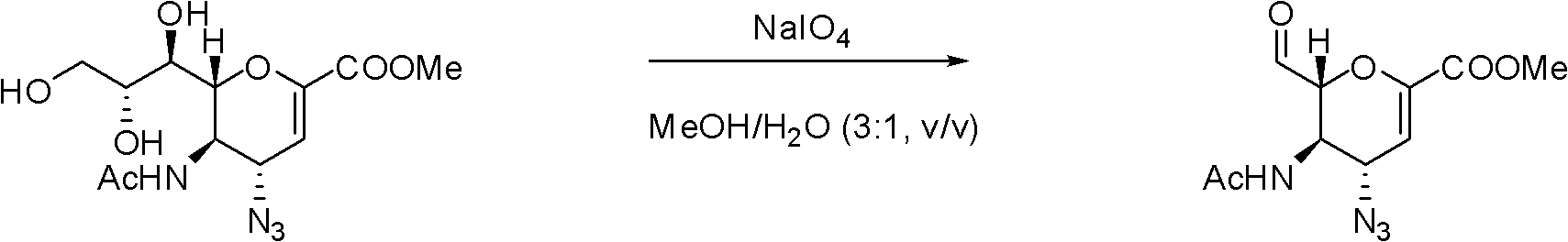Sialic acid analog and application thereof to preparation of anti-influenza-virus medicaments
An analogue, sialic acid technology, applied in the field of chemistry, can solve problems such as poor bioavailability and rapid excretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0046] Preparation Example 1: Preparation of (3R, 4S)-3-acetamide-4-azido-2-formaldehyde-3,4-dihydro-2H-pyran-6-carboxylic acid methyl ester a) (4S, 5R) - Preparation of 5-acetylamino-4-azido-6-formyl-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester
[0047] For the preparation method, see J.CHEM.PERKIN TRANS I 1995.1173-1180.
[0048] b) Preparation of (3R, 4S)-3-acetamide-4-azido-2-carbaldehyde-3,4-dihydro-2H-pyran-6-carboxylic acid methyl ester
[0049]
[0050] The compound N-acetyl-2,4-dideoxy-2,3-dehydro-4α-azido-D-neuraminic acid methyl ester was added to methanol / water (v / v=3:1) mixture , stirred in an ice-water bath until completely dissolved, and slowly added NaIO 4 Afterwards, allow the temperature to rise slowly to room temperature and continue to stir for about 16 hours. TLC (dichloromethane / methanol=9:1, v / v) traces the disappearance of the raw material. Add anhydrous methanol after evaporating the solvent, and use anhydrous magnesium sulfate After dryin...
Embodiment 1
[0052] Example 1: Preparation of (2S, 3R, 4S)-3-acetylamino-4-amino-2-(piperidine-1-methyl)-3,4-dihydro-2H-pyran-6-carboxylic acid
[0053] a) (2S, 3R, 4S)-3-acetylamino-4-azido-2-(piperidine-1-methyl)-3,4-dihydro-2H-pyran-6-carboxylic acid methyl ester preparation of
[0054] (3R,4S)-3-acetamide-4-azido-2-formyl-3,4-dihydro-2H-pyran-6-carboxylic acid methyl ester 200mg (0.75mmol) and hexahydropyridine 76.5mg (0.9mmol) into a 25ml round bottom flask, add 5ml of anhydrous methanol, stir at room temperature under nitrogen protection until the solid dissolves, add 1 drop of glacial acetic acid, continue stirring for 10min, add NaBH 3 CN 57mg (0.9mmol), stirred at room temperature for 16 hours, TLC (dichloromethane / methanol=9:1, v / v) tracked the disappearance of raw materials, added a small amount of saturated NaHCO 3 Aqueous solution, after extraction with dichloromethane, dried over anhydrous sodium sulfate, and the solvent was evaporated by rotary evaporation to obtain a ye...
Embodiment 2
[0068] Example 2: (2S, 3R, 4S)-3-acetylamino-4-amino-2-(4-hydroxypiperidine-1-methyl)-3,4-dihydro-2H-pyran-6- Preparation of formic acid
[0069] a) (2S, 3R, 4S)-3-acetylamino-4-azido-2-(4-hydroxypiperidine-1-methyl)-3,4-dihydro-2H-pyran-6- Preparation of methyl formate
[0070] compound
[0071] (2S, 3R, 4S)-3-Acetamido-4-azido-2-(4-hydroxypiperidine-1-methyl)-3,4-dihydro-2H-pyran-6-carboxylic acid The ester is implemented with reference to the intermediate (3R, 4S)-3-acetamide-4-azido-2-formyl-3,4-dihydro-2H-pyran-6-carboxylic acid methyl ester and 4-hydroxypiperidine Obtained by the experimental operation of Example 1(a), the yield was 22.6% under silica gel column chromatography purification conditions (dichloromethane / methanol=13:1, v / v).
[0072] ESI-MSm / z: 354.4 (M+H) +
[0073] 1 H-NMR (400MHz, CDCl 3. )δppm 6.24-6.22 (d, NH, J=8.2Hz) 5.98-5.97 (d, 3-CH, J=12.4Hz), 4.36-4.22 (m, 6-CH), 4.17-4.15 (m, NCH 2 CH 2 C H O), 3.80(s, COOC H 3 ), 3.68-3.66 (m, 5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com