Canine parvovirus-like particles and preparation method and application thereof
A canine parvovirus and virus-like technology, applied in the field of microorganisms and veterinary biopharmaceuticals, can solve the problems of virulence reversion and recombination, failure to cause cellular immune response, weak immunogenicity of inactivated vaccines, etc., to achieve good immunity and prevention Efficiency, ease of large-scale production, and high immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
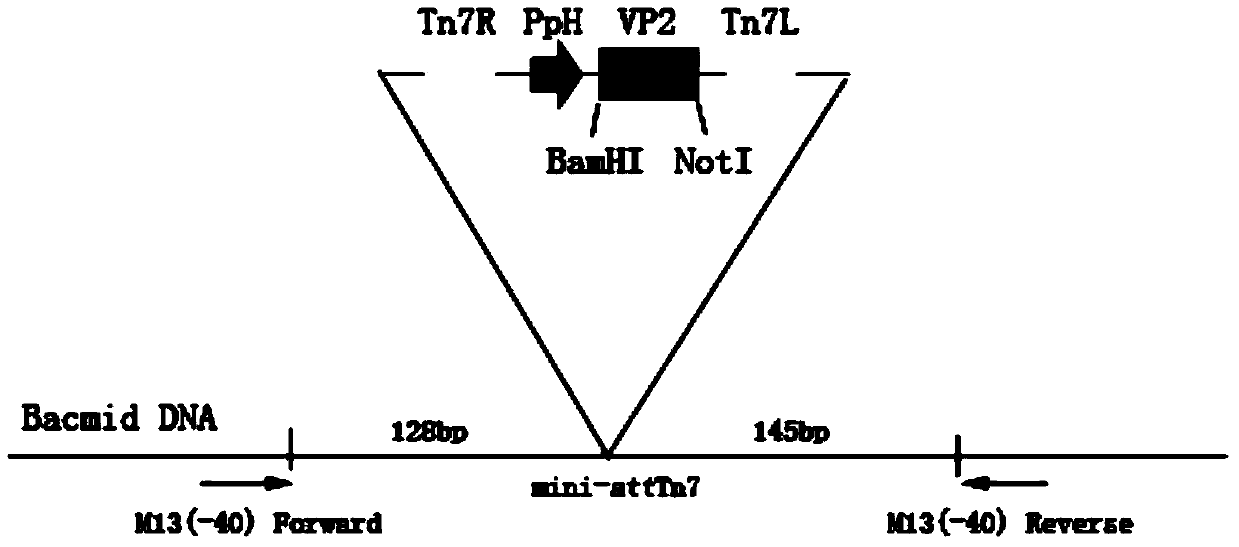
[0024] Example 1 Preparation and identification of canine parvovirus virus-like particles
[0025] 1 material
[0026] The donor plasmid pFastBac1 was purchased from Invitrogen, and the strain E.coliDH10Bac, insect cell Sf9, and canine parvovirus were provided by Changchun Sino Biotechnology Co., Ltd.
[0027] pEASY-bluntsimple, IPTG, and X-gal were purchased from Dalian Bao Biological Company, Phusion DNA polymerase, T4DNA ligase, and restriction enzyme were purchased from NEB Company, competent cells were purchased from Takata Company, genomic DNA extraction kit, gel Recovery kits and plasmid extraction kits were purchased from Axygen, liposome Lipofectin2000 was purchased from Invitrogen, ampicillin, tetracycline, kanamycin, and gentamicin were purchased from Sigma, and fetal bovine serum was Changchun West Produced by Nuo Biotechnology Co., Ltd., TC-100 insect cell culture medium was purchased from Applichem Company, rabbit anti-canine parvovirus polyclonal antibody was p...
Embodiment 2
[0061] Preparation of Example 2 Canine Parvovirus Enteritis Vaccine and Animal Immunization Test
[0062] 1 material
[0063] After the aluminum phosphate was prepared to an appropriate concentration with isotonic saline, it was ultrasonically crushed for 20 minutes to prepare a suspension, which was sterilized at high temperature for later use. Female guinea pigs (about 250g), Chinese pastoral dogs (2-4 months old, CPV hemagglutination inhibitory antibody titer less than 1:2 3 ) were purchased from Changchun Institute of Biological Products.
[0064] 2 methods
[0065] 2.1 Preparation of canine parvovirus enteritis vaccine
[0066] Inoculate Sf9 cells with recombinant baculovirus expressing canine parvovirus VP2 gene, and culture continuously at 27°C for 4-5 days. After the cells become lesions, harvest the cell suspension, freeze and thaw three times, centrifuge at 2000rpm for 10min, and harvest the supernatant , to obtain canine parvovirus virus-like particles. Canine ...
Embodiment 3
[0082] Example 3 The protective test of canine parvovirus enteritis vaccine to dogs
[0083] 1 material
[0084] Canine parvovirus enteritis vaccine was prepared according to the method described in Example 2, and the Chinese pastoral dog (2-4 months old, CPV hemagglutination inhibitory antibody titer was less than 1:2 3 ) were purchased from Changchun Institute of Biological Products.
[0085] 2 methods
[0086] 2.1 Animal immunity
[0087] Take 12 Chinese pastoral dogs, which are labeled M1, O2, O3, and C4 in turn, and grouped and immunized according to Table 3 in Example 2. Among them, the M1 group received intramuscular injection, and the O2 and O3 groups received oral immunization, with the first immunization on the 0th day and the booster immunization on the 15th day.
[0088] 2.2 Animal infection
[0089] Canine parvovirus virulence challenge was performed 21 days after immunization. The specific method is as follows: oral inoculation of canine parvovirus virulenc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com