Recombined chimeric antibody against human tumor necrosis factor alpha
A tumor necrosis factor, chimeric antibody technology, applied in the field of immunology, can solve problems such as attack
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Production of anti-hTNFα mouse monoclonal antibody
[0084] Four-week-old BALB / c mice were subcutaneously injected with 20 μg of recombinant hTNFα (rhTNFα, purchased from PeproTech Inc.) in complete adjuvant. Five injections are given every three to four weeks. Finally, a single injection of 20 μg rhTNFα was given intraperitoneally. After serum testing, mice with high levels of anti-hTNFα antibody serum were identified. The mouse spleen was taken out and fused with the mouse myeloma Sp2 / O cell line. Mix 5×10 8 Sp2 / O cells with 5 × 10 8 Splenocytes were fused in a solution of 50% polyethylene glycol (PEG, molecular weight 1450) and 5% dimethylsulfoxide (DMSO). Use Iscove medium (containing 10% fetal bovine serum, 100 units / ml penicillin, 100 μg / ml streptomycin, 0.1 mM hypoxanthine, 0.4 μM aminopterin and 16 μg thymidine) to adjust the number of spleen cells to 7.5×10 5 / ml, add 0.2ml into the wells of 96-well culture plate. Place at 37°C 5% CO 2 in the ...
Embodiment 2
[0085] Qualitative Example 2 Anti-hTNFα murine antibody:
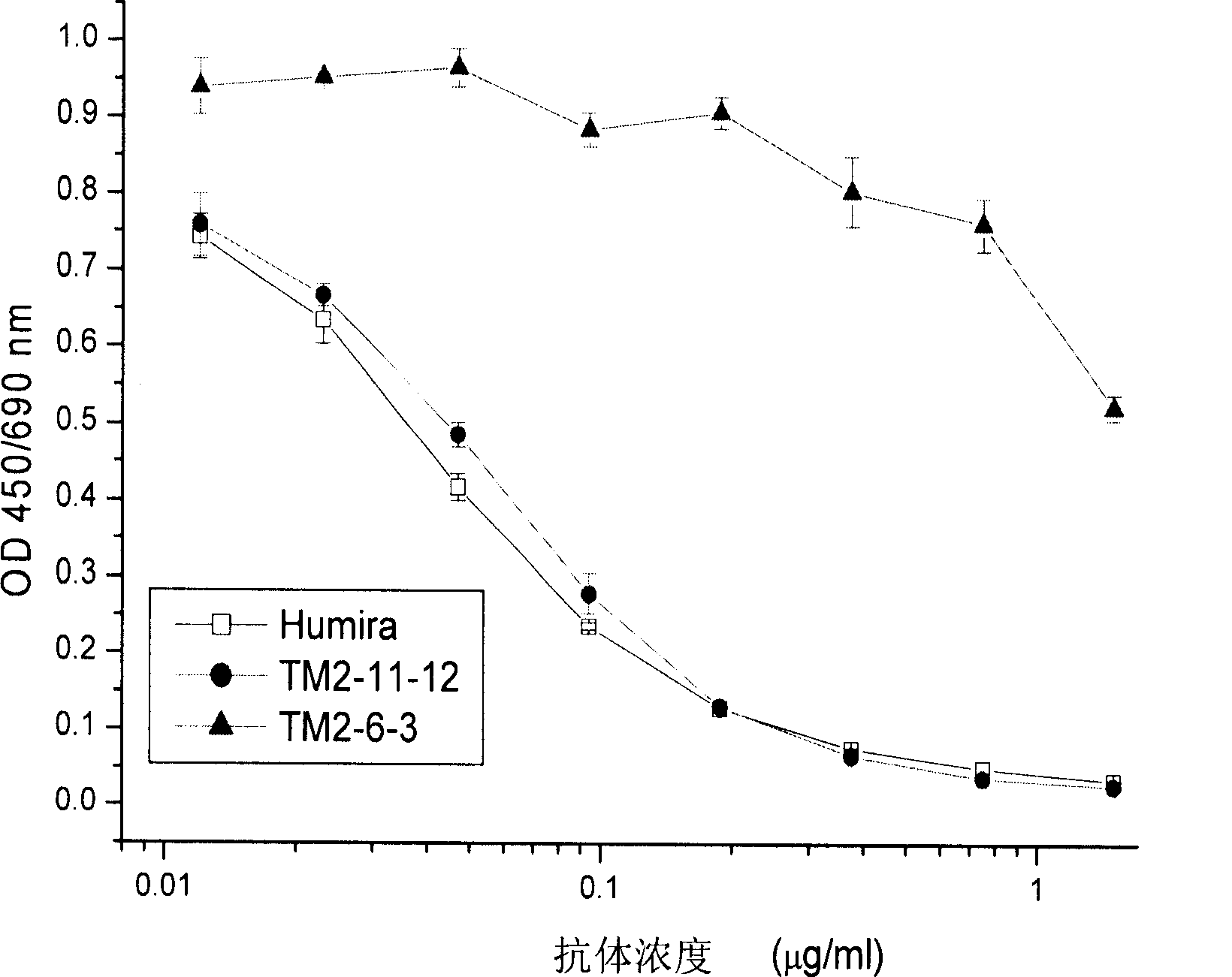
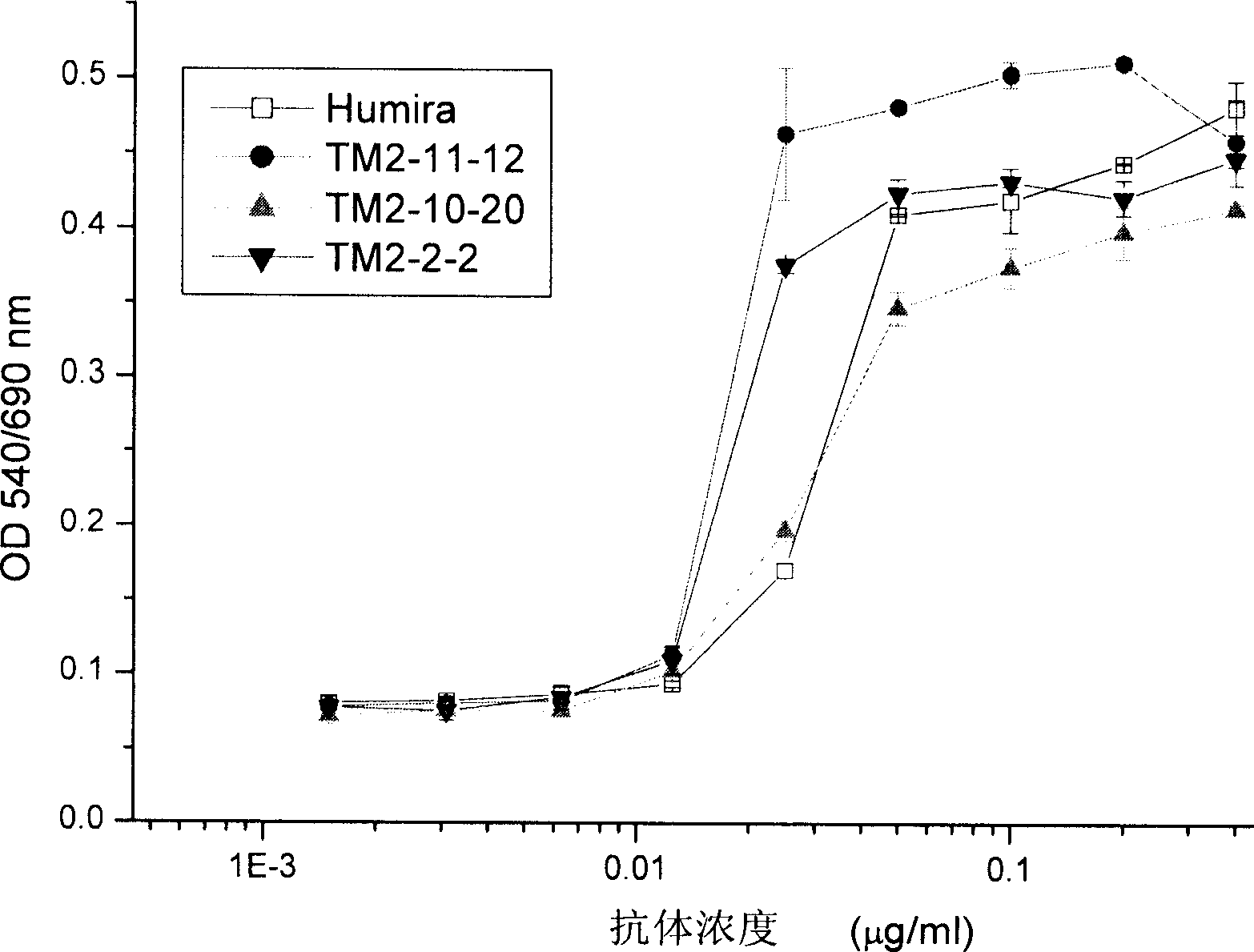
[0086] There are two assays for the characterization of anti-hTNFα antibodies. One method measures the competitive binding of the antibody to hTNFα for Humira, and the other method measures the ability of the antibody to neutralize hTNFα in a L929 cytotoxicity assay. The two methods and their experimental results are described respectively below.
[0087] 1. Competition Binding Assay with Humira Antibody
[0088] Horseradish peroxidase (HRP, Boehringer Manheim)-labeled anti-hTNFα human antibody Humira (Abbott) was used as a reagent. rhTNFα (50 μl, 0.05 μg / ml) was coated on a microtiter plate (CorningLife Sciences) and left overnight at room temperature. The coating solution was discarded, the wells were blocked with 1% skim milk dissolved in phosphate buffered saline (PBS) for 0.5 hours, and the wells were washed with PBS containing 0.05% Tween 20. Then, a mixture of 50 μl of growth medium (DMEM+5% FBS, Invitrogen)...
Embodiment 3
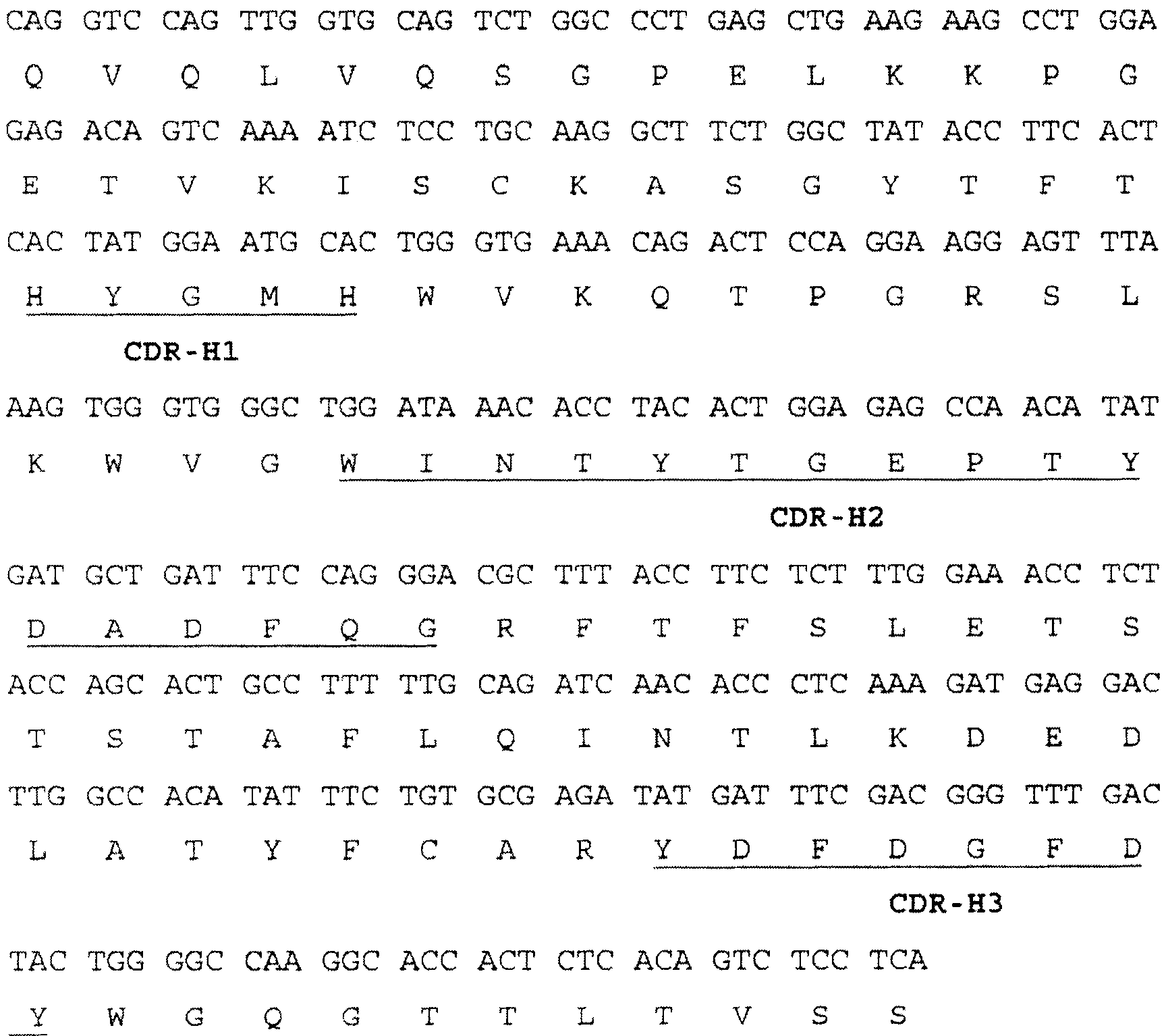
[0091] Example 3 Cloning of heavy chain and light chain of TM2-11-12 murine antibody
[0092] In order to express the C2-11-12 chimeric antibody, the DNA fragments encoding the heavy and light chain variable regions of the anti-hTNFα murine antibody TM2-11-12 must first be obtained. RNA was isolated from TM2-1112 mouse hybridoma cells using an RNA purification kit (Invitrogen Corp.) to prepare cDNA (GeneRacer kit, Invitrogen Corp.). The heavy chain was isolated from cDNA by polymerase chain reaction (PCR) using a 5' primer (5'CGACTGGAGCACGAGGACACTGA-3', SEQ ID NO: 11) and a 3' primer (5'TCCAGGGGCCAGTGGATAGACAGA-3', SEQ ID NO: 12) Variable region DNA fragments. The 3' primer is homologous and antisense to the mouse igG1 heavy chain constant region. These resulting DNA fragments were cloned into TOPO TA vector (Invitrogen) and sequenced. All heavy chain variable region clones showed the same nucleotide sequence (SEQ ID NO: 1). The nucleotide sequence and its encoded amino ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com