SM-protein based secretion engineering
A protein and heterologous protein technology, applied in genetic engineering, peptide/protein components, animal/human proteins, etc., can solve problems such as limited understanding of basic regulatory mechanisms, and achieve the effect of reducing costs and improving protein yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0277] Example 1: Localization of Sly1 and Munc18c in HEK-293 along the secretory pathway.
[0278] We used RT-PCR based analysis to characterize the expression of SM proteins Sly1 and Munc18 isoforms (a, b, c) in HEK-293. as in figure 1 As shown in a and 1b, sly1 (NM_016160) and munc18c (NM_007269) were expressed at high levels, muc18b (NM_006949) was expressed in a small amount, and the transcript of neuron-specific munc18a (NM_003165) could not be detected. By Western blotting ( figure 1 c) To confirm the SM protein profile. Sly1 and Munc18 were analyzed by co-expression of YFP-Sly1 (pRP32) and CFP-syntaxin 5 (pRP40) or YFP-Munc18c (pRP23) and CFP-syntaxin 4 (pRP29) in HEK-293. Intracellular localization of I-type Munc18c. Syntaxin 5 is a SNARE that binds to Sly1 localized in the Golgi apparatus, and syntaxin 4 is a SNARE that interacts with Munc18c bound to the cell membrane. Confocal microscopy showed that Sly1 showed extremely dense perinuclear co-localization wit...
Embodiment 2
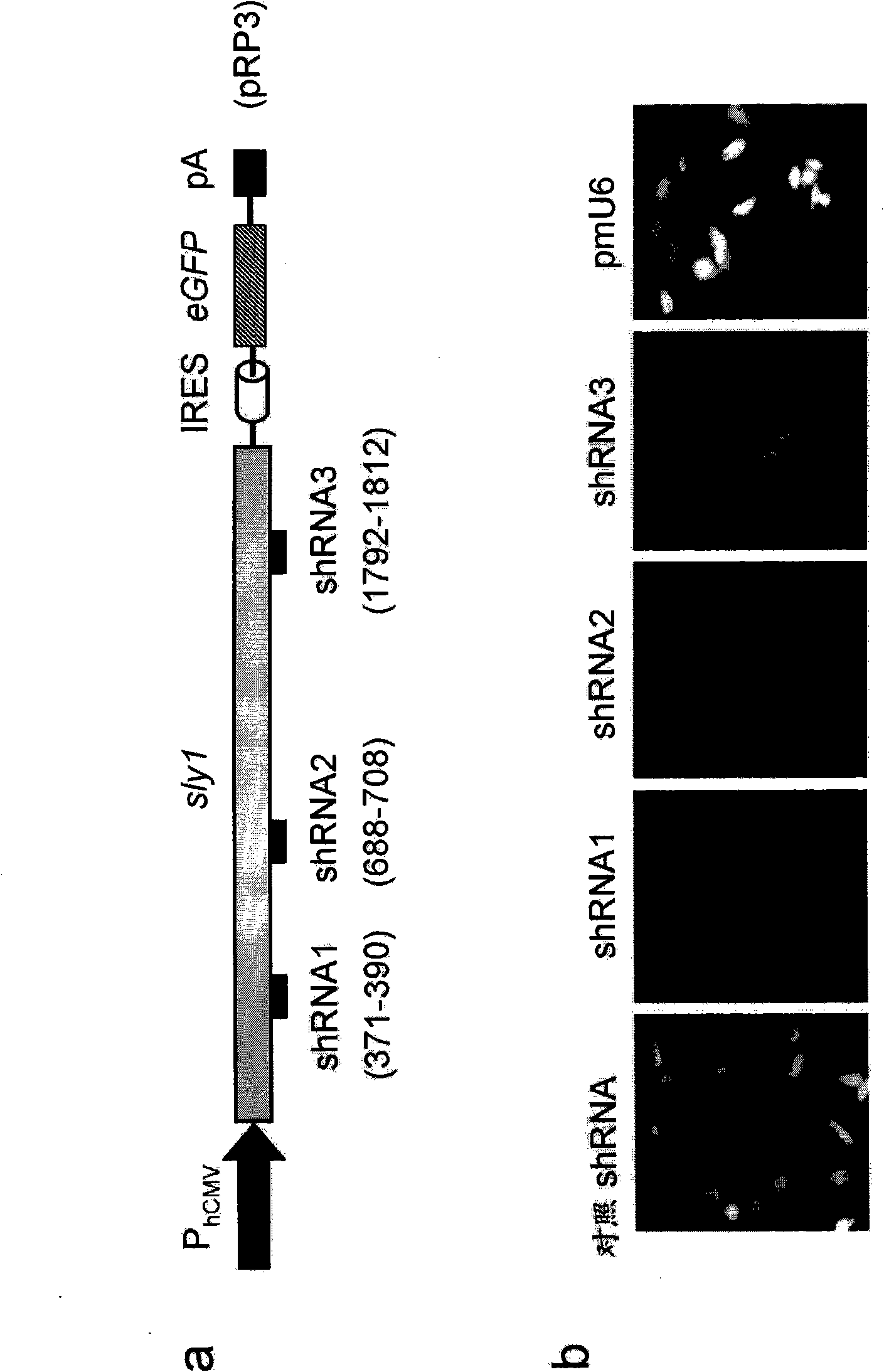
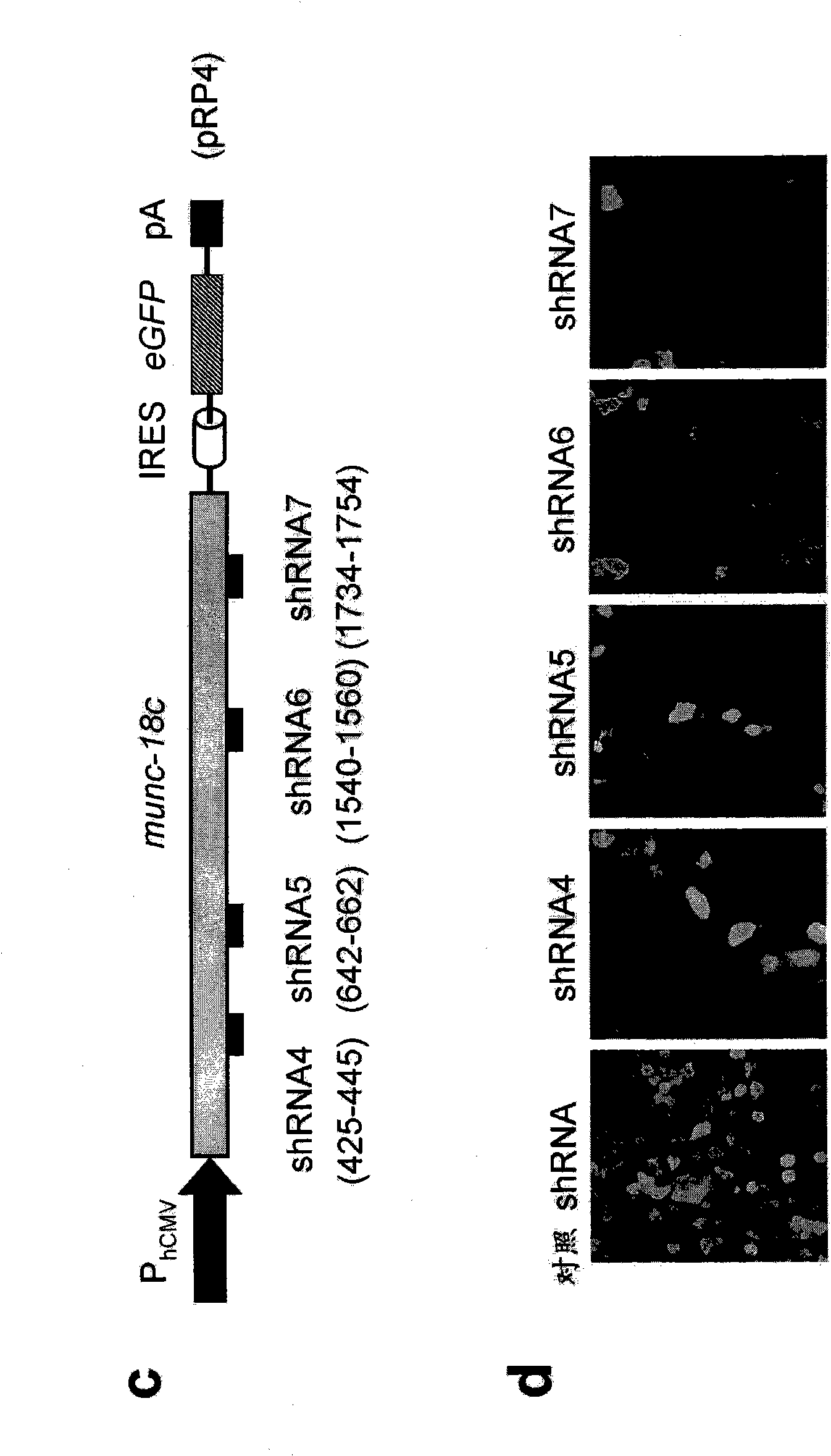
[0279] Example 2: Sly1 and Munc18 regulate protein secretion.
[0280] SM proteins are known to control vesicle fusion necessary for intracellular protein transport, but their role for protein secretion remains unknown. To characterize the effect of Sly1 and Munc18 on overall exocytosis, we designed shRNAs specific to these SM proteins. Knockdown of Sly1 and Munc18c was demonstrated by the following method: cells were encoded with bicistronic Sly1 (pRP3; P hCMV -sly1-IRES-eGFP-pA) and encoding Munc18c (pRP4; P hCMV -munc 18c-IRES-eGFP-pA) reporter construct was co-transfected with specific and non-specific control shRNA, and fluorescent microscopy was performed ( figure 2 ). The ability of each shRNA to knock down endogenous Sly1 and Munc18c expression by up to 70% was demonstrated in HEK-293 ( image 3 a and 3c). To analyze the effect of Sly1 and Munc18c knockdown on the overall protein secretion ability of mammalian cells, we combined pSEAP2 control and pRP5 (shRNA sly...
Embodiment 3
[0281] Example 3: Ectopic expression of Sly1 and Munc18c improves the secretory ability of mammalian cells.
[0282] Ectopic expression of Sly1 or Munc18c in CHO-K1 ( Figure 4 a After 4b, 4c), SEAP, SAMY or VEGF 121 Heterologous production was increased up to 5-fold, which is consistent with the promoter used to initiate transcription of the product gene (P SV40 ,P hCMV ,P EF1α ) is irrelevant. Similar results were also observed when using HEK-293 cells (data not shown). Because the mRNA levels of SEAP, SAMY, and VEGF were essentially constant with or without elevated Sly1, Munc18c, or both ( Figure 4 d), so the increase in heterologous protein production is mediated by post-translational mechanisms. Our results are consistent with previous studies arguing that the Munc18 protein inhibits exocytosis in a range of cell types, including adipocytes and muscle cells (Riento et al., 2000; Kanda et al., 2005; Tellam et al., 1997; Thurmond et al. et al., 1998), which is the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com