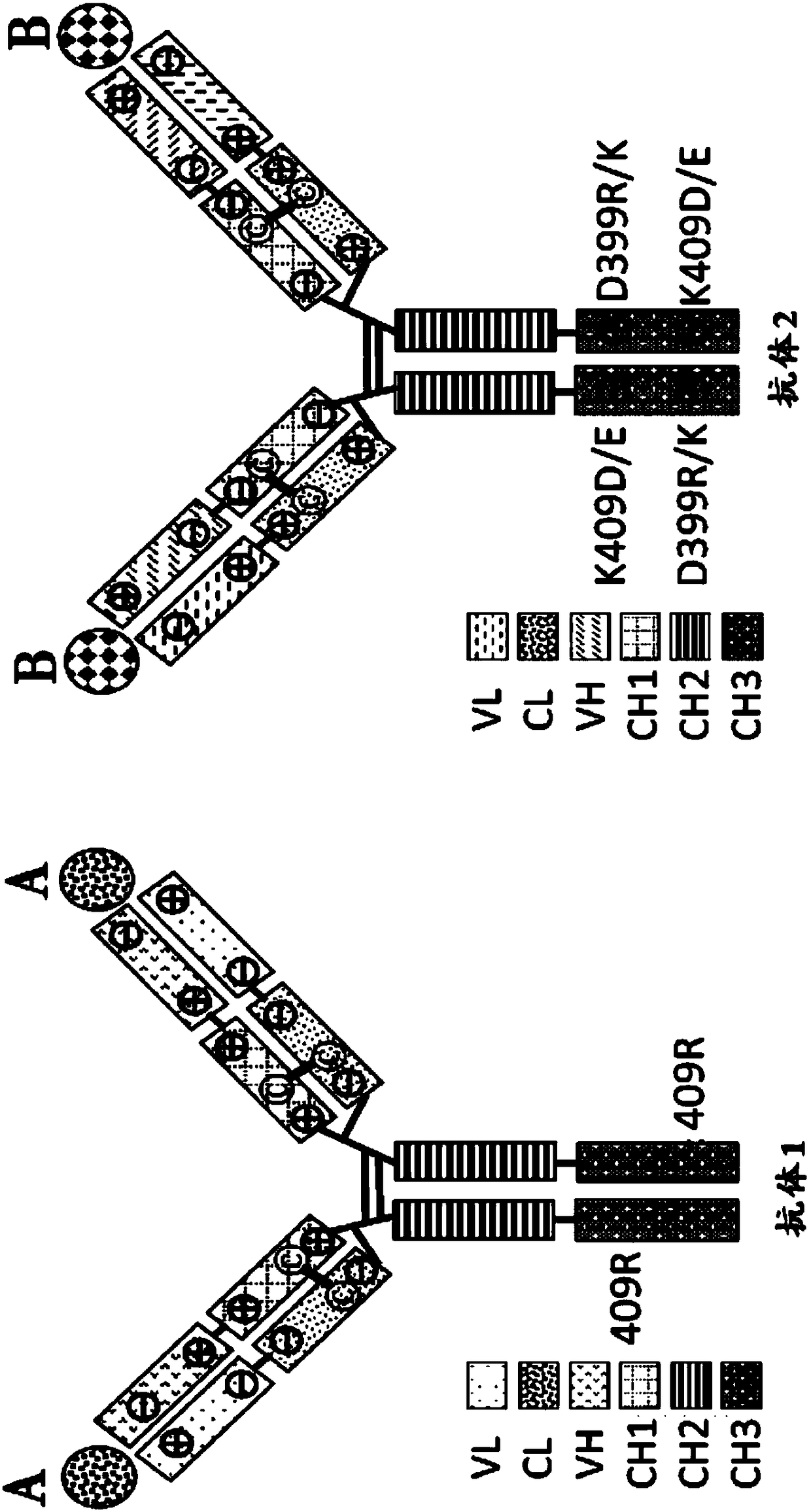
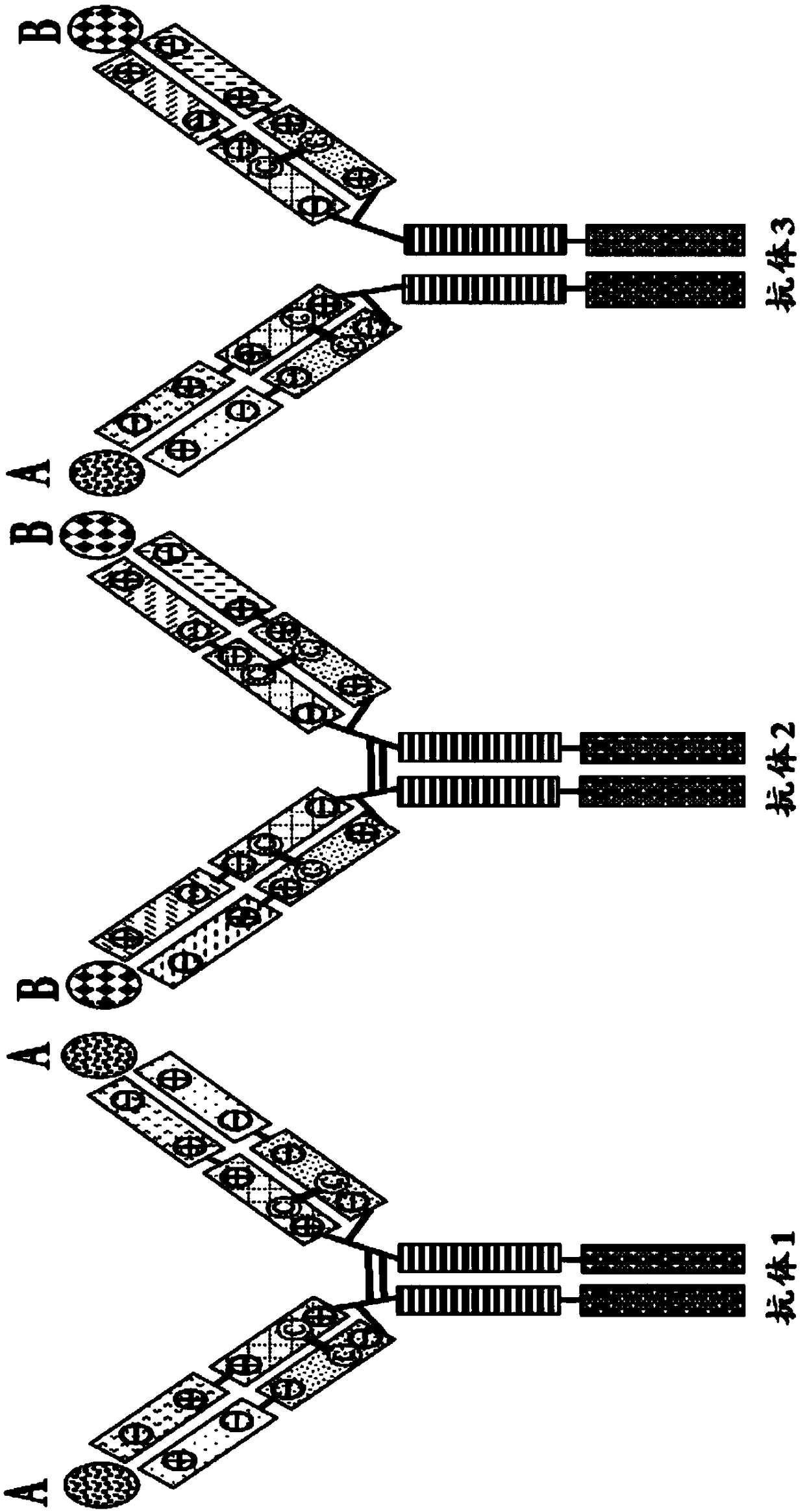
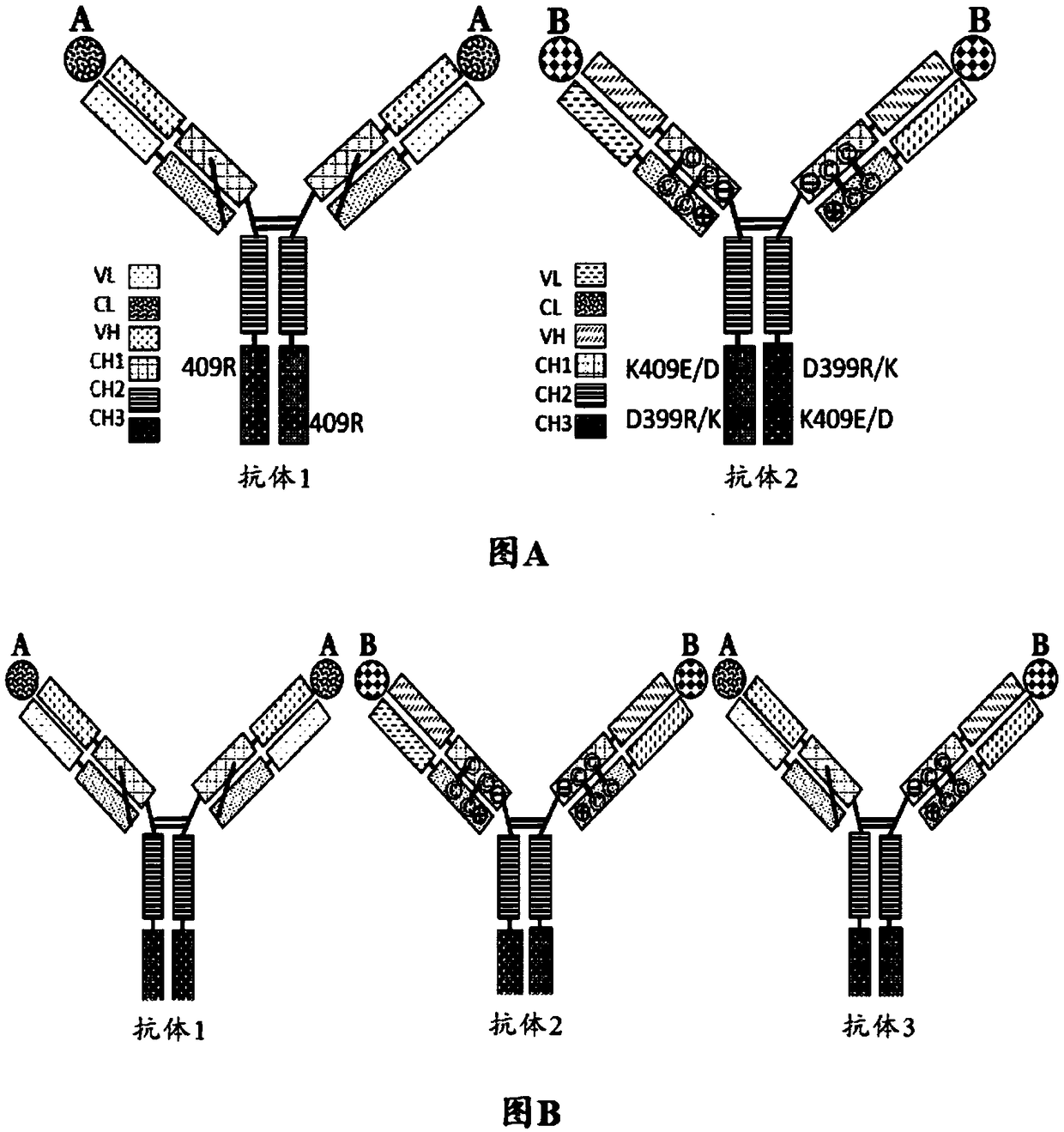
Mixtures of antibodies
A technology of antibody mixture and mixture, applied in the direction of antibodies, antibody medical components, anti-animal/human immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0569] Example 1: Designing HC Partner Orientation Changes and LC Partner Orientation Changes
[0570] Preventing non-cognate HC / LC pairing would greatly limit the number of antibody species produced by host cells transfected with DNA encoding multiple full-length antibodies. see for example Figure 4 . To ensure that only homologous LC / HC pairs form, it is critical to find efficient ways to control the kinetics of the HC / LC assembly process such that each LC strongly favors pairing with its cognate HC and disfavors pairing with noncognate HC. Since both the VH / VL interface and the CH1 / CL interface are involved in HC / LC recognition and engagement (see Knarr et al. (1995), J. Biol. Chem. 270:27589-27594; Feige et al. (2009), Mol. Cell 34 (2004), J.Mol.Biol.342:665-679; Rothlisberger et al. (2005), J .Mol.Biol.347:773-789, all of which are incorporated herein by reference in their entirety), so both interfaces were engineered to force homologous HC / LC pairing, as explained in...
Embodiment 2
[0587] Example 2: Design and testing of disulfide bridges added in the HC / LC interface
[0588] We hypothesized that changes that generate additional HC / LC interchain disulfide bridges might enhance the association of homologous HC / LC pairs. In unmodified antibodies, interchain disulfide bonds are solvent-exposed, while intra-chain disulfide bonds are embedded between two antiparallel β-sheet structures within each domain, ie, are not exposed to solvent. For example, the interchain disulfide bonds in the hinge region are solvent exposed, as are the disulfide bonds between HC and LC. Cysteine residues exposed to solvent were found to be more reactive than unexposed cysteine residues. Therefore, when performing cysteine substitutions, we try to generate disulfide bridges that are partially or fully solvent exposed.
[0589] The introduced disulfide bridge has been shown to stabilize the Fv fragment by stabilizing the VH / VL interaction, resulting in higher yield and solub...
Embodiment 3
[0609] Example 3: Testing Antibody Cocktails Containing Altered LC Partner Orientation and Altered HC Partner Orientation That Are Charge Pair Substitutions and / or Cysteine Substitutions
[0610] The identity of many residue contact pairs in HC and LC suitable for charge pair substitutions or cysteine substitutions (Example 1 and Example 2) has been determined by analysis of published tertiary structures and has been performed A number of cysteine substitutions were tested and a number of such substitutions and their combinations were made and tested to determine their effect on homologous HC / LC pairing. To quickly assess the effectiveness of various LC partner orientation changes and HC partner orientation changes in forcing homologous HC / LC pairing, a "chain-off" experiment was performed as described below.
[0611] Antibody-encoding DNA constructs were prepared using methods similar to those described in Example 2. A DNA fragment encoding the human IgG1 HC of the hum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com