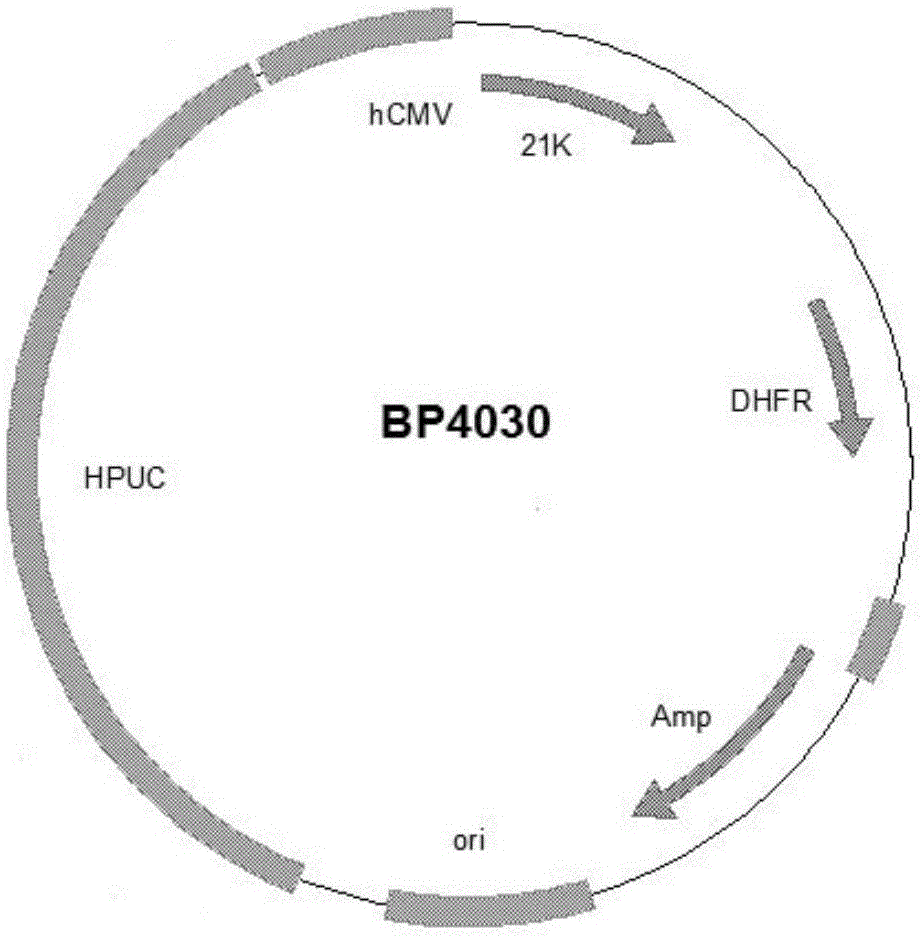
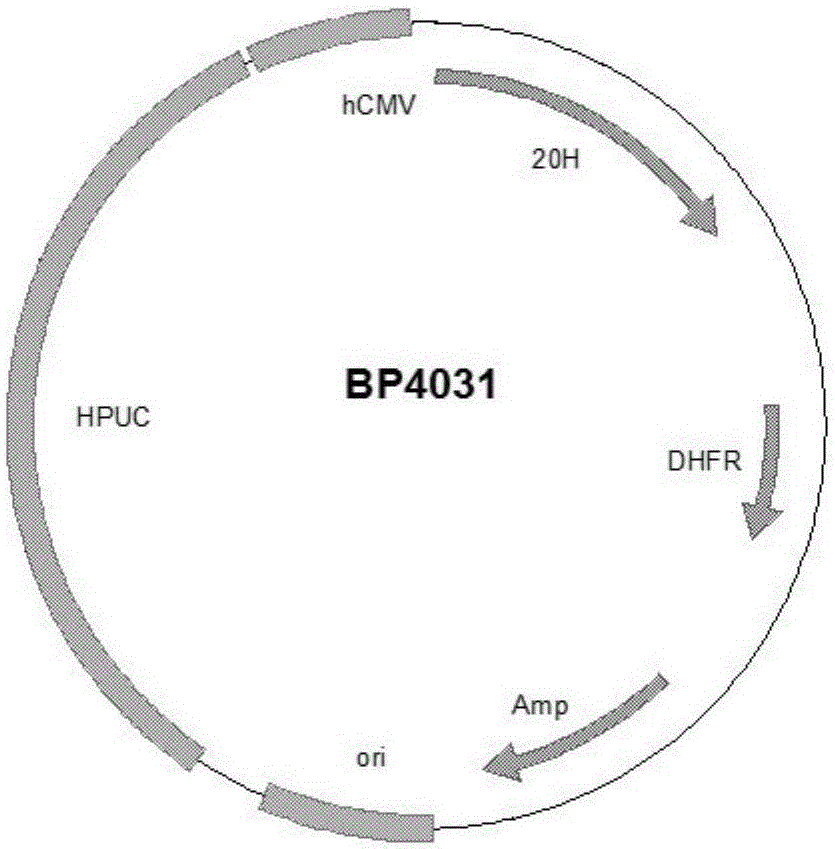
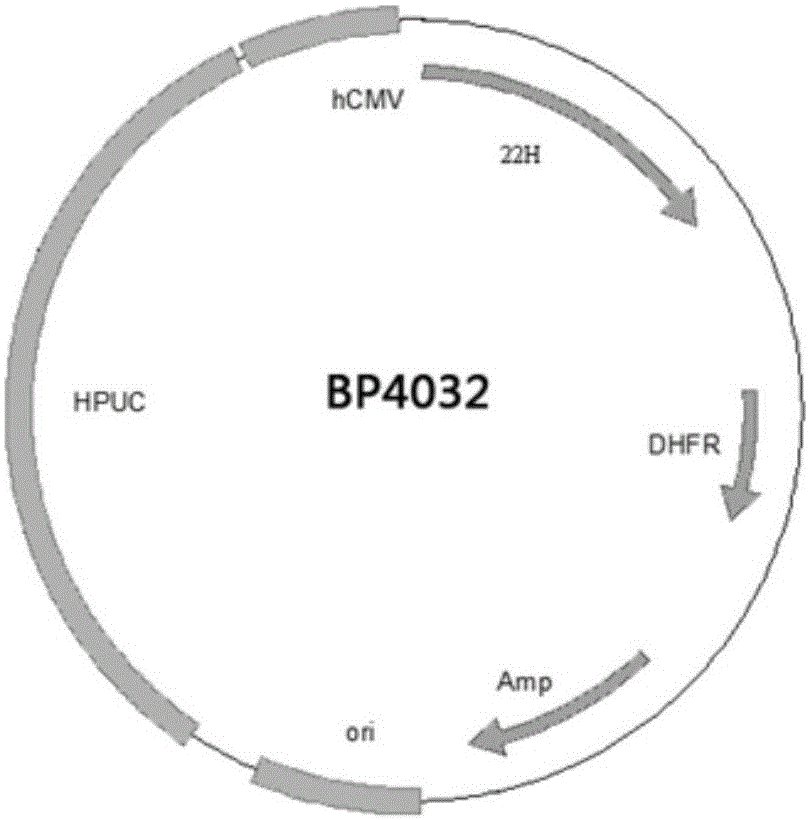
Preparation and application of anti-human PCSK9 (pro-protein convertase subtilisin/kexin 9) antibody
An antibody and chimeric antibody technology, applied in the field of genetic engineering, can solve the problem that the lipid level cannot reach the expected target, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of anti-hPCSK9 chimeric antibody
[0033] 1. Obtaining the sequence of anti-hPCSK9 monoclonal antibody
[0034] 1.1 hPCSK9 immunized mouse hybridoma cell line was obtained
[0035] Mix equal volumes of hPCSK9 and quickantibody-mouse3W immune adjuvant (KX0210041, Beijing Kang Biquan Biotechnology Co., Ltd.), the final concentration of PCSK9 is 200 μg / ml, immunize Balb / c cells, and use intramuscular injection, 100 μl / mouse, to obtain immune spleen cells . The immune splenocytes were fused with Sp / 20 (mouse myeloma cells), selectively cultured by HAT, cultured in a semi-solid medium, and anti-hPCSK9 positive hybridoma cell lines were screened out, and then a subcloning screening was performed to finally obtain a cell line capable of Monoclonal hybridoma cell line (4-2B4-4 cell line) secreting anti-hPCSK9 antibody protein.
[0036] 1.2 Obtaining the variable region sequence of anti-hPCSK9 antibody
[0037] Using the anti-PCSK9 antibody monoclonal c...
Embodiment 2
[0057] Example 2 Chimeric Antibody Binding Affinity to Human PCSK9
[0058] The binding affinity of the chimeric antibody shown in the present invention to human PCSK9 is determined by biomembrane interference technology. First, human PCSK9 (R&D Corporation) was biotinylated to obtain biotinylated human PCSK9. Then in OtectQK e (PallFortebio) protein molecular interaction instrument, immobilize it on the SAbiosensor sensor (Pall company), and after equilibrating in the same buffer, transfer to samples containing chimeric antibody in serial three-fold dilution (600nM to 0.82nM) Binding-dissociation reactions are carried out in the wells. Using Octet software to record the response signal and calculate the binding kinetic parameters, summarized in Table 1, the data are expressed as Mean±SD.
[0059] Table 1. Kinetic parameters of chimeric antibody binding to PCSK9
[0060]
Embodiment 3
[0061] Example 3 Chimeric Antibody Biological Activity Determination
[0062] 1. Effect of chimeric antibody on PCKS9 degradation of hepatic LDLR
[0063] Human liver cancer HepG2 cells were cultured to the logarithmic growth phase, the cells were washed once with PBS, digested with trypsin to make a cell suspension, and the cell density was adjusted to 1×10 6 cells / ml. The cells were seeded in a 96-well culture plate (100 μl / well), and 200 μl of medium was added to the edge wells. Place the culture plate at 37°C, 5% CO 2 Incubate for 24 hours in the incubator. Remove the cell culture supernatant, add 100 μl serum-free cell culture medium to each well, and continue culturing for 16 hours; human PCSK9 (25 μg / ml) and 100 nM, 75 nM, 50 nM, 25 nM, 12.5 nM, 6.25 nM chimeric antibody were added in serum-free h -Pre-incubated in DMEM medium for 2 hours, added to the cell wells cultured without serum, placed in 37°C, 5% CO2 incubator and incubated for 6 hours, used for LDLR immuno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com