Preparation method of allergen-specific IgE antibody composite quality control product and allergen-specific IgE antibody composite quality control product
An allergen and specific technology, applied in the field of genetic engineering, can solve the problems of easy deterioration or degradation, insufficient supply, cumbersome and difficult adjustment of IgE antibody mixed debugging, and achieve the effect of good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A preparation method of allergen-specific IgE antibody composite quality control product, comprising the following steps:
[0026] S1: Prepare allergen-specific IgE antibodies, as follows: Purify the collected allergen-positive serum with allergen-specific IgE antibody affinity chromatography, elute and collect human IgE; then use the above-mentioned allergen-specific IgE antibodies Affinity chromatography purification of high-purity allergens, elution and collection of allergen-specific IgE antibodies;
[0027] Separation and purification can also be carried out by electrophoresis, salting-out precipitation or other techniques. Total IgE antibodies can be obtained by first purification, and then allergen-specific IgE antibodies can be purified to prevent the mixing of IgG antibodies;
[0028] S2: obtaining the target gene, obtaining the constant region gene sequence and the variable region gene sequence of human IgE;
[0029] The purified allergen-specific IgE antibod...
Embodiment 1
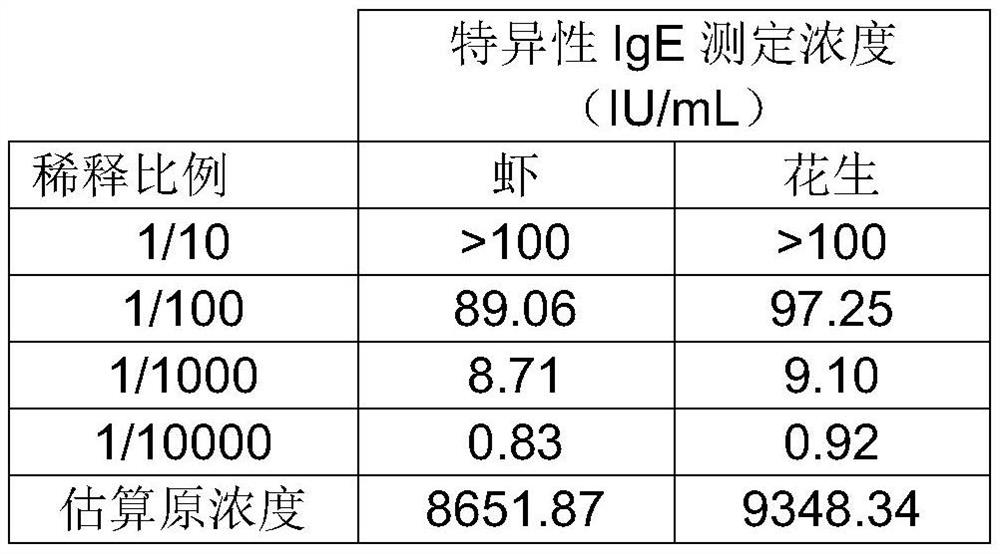
[0048] This example takes the preparation of the composite quality control of shrimp-peanut allergen-specific IgE antibody as an example.
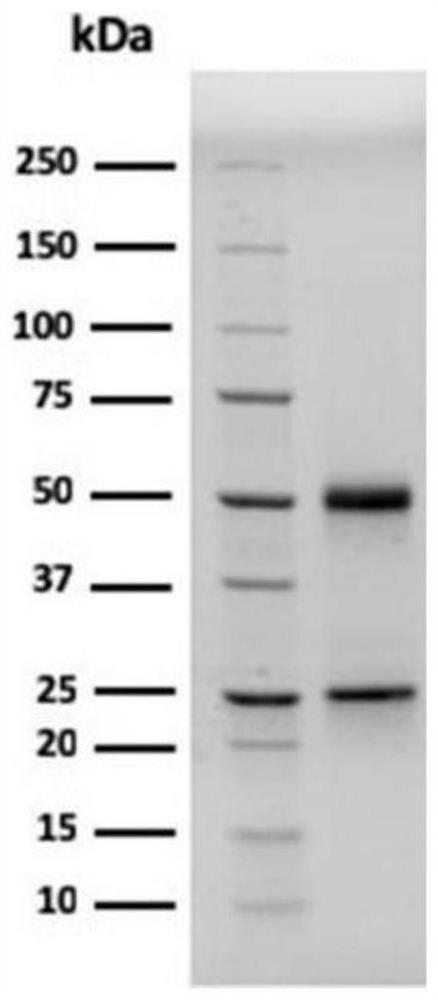
[0049] 1. Preparation of high-purity shrimp-peanut allergen-specific IgE antibody: select one case each of shrimp allergen-positive samples and peanut allergen-positive samples purchased from PlasmaLab in the United States, and mix them with binding buffer (0.02MPBS, pH7.4) volume ratio For 1:1 mixing, 0.8μm membrane filter to remove fibrinogen (to prevent clogging the column). Use 5-10 times the volume of binding buffer to pass through the anti-human IgE antibody column; then load the prepared serum sample, wash the column with binding buffer, and elute the impurity protein; then elute with glycine eluent, and collect at the same time Transudate (about 3-4ml / tube) until there is no protein in the transudate; measure the protein content in each collection tube, combine the protein tubes to obtain the shrimp-peanut allergen-specific IgE ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com