Recombinant virus of chimeric IBV H120 S1 gene ectodomain suitable for cell culture and construction method and application thereof
A technology of IBVH120S1 and recombinant virus, applied in the direction of microorganism-based methods, medical raw materials derived from viruses/bacteriophages, antiviral agents, etc., can solve the problems of exogenous virus pollution costs, small toxin production, long production cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
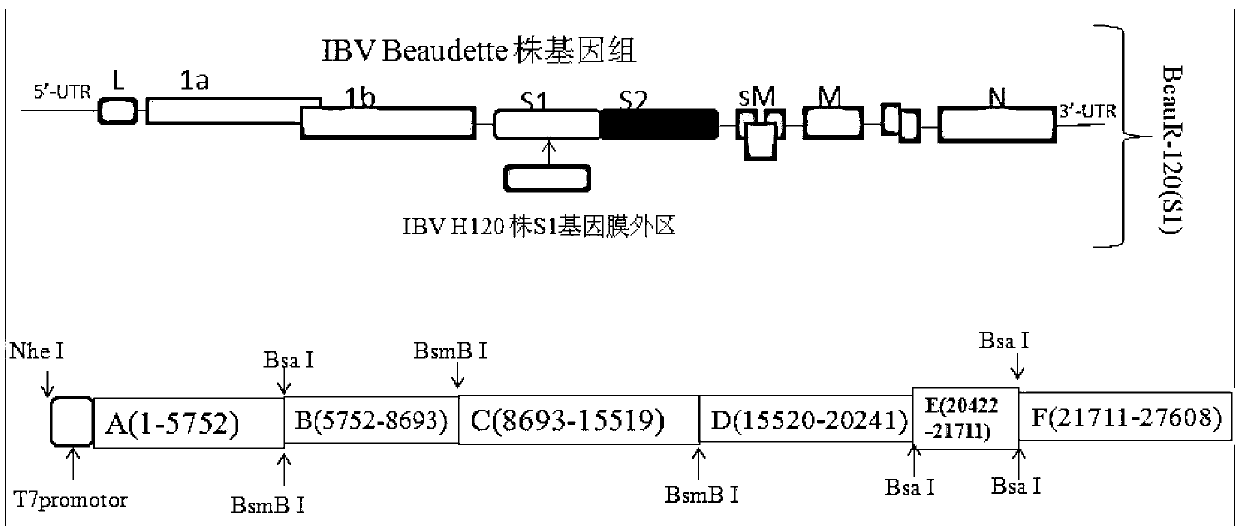
[0053] Example 1 Construction of IBV recombinant virus BeauR-H120 (S1) of the present invention
[0054] The specific construction method is completed by the following steps:
[0055] 1. Use Trizol reagent (Invitrogen, USA) to extract total RNA from IBV H120 vaccine strain and Beaudette P65 generation cytotoxicity respectively. The downstream primers in Table 1 were used for reverse transcription, and the primer Beau-H21711R was used for reverse transcription of the S1 gene extra-membrane region of IBV H120 strain. The primers Beau-5752R, Beau-8693R, Beau-15520R, Beau-20422R, Beau-27608R are used for reverse transcription of the backbone fragment of the parent virus Beaudette strain.
[0056] Table 1 Primer pairs used in the construction of the recombinant virus BeauR-H120 (S1)
[0057]
[0058] 2. Using the primer pair Beau-H20421F and Beau-H21711R in Table 1 to amplify the S1 gene extra-membrane fragment of IBV H120 strain, the resulting fragment was labeled H120(S1e). Use other 5...
Embodiment 2
[0073] Example 2 Cultivation of IBV recombinant virus BeauR-H120 (S1) of the present invention
[0074] 1 method
[0075] 1.1 Stable passage of successfully rescued IBV recombinant virus
[0076] The successfully rescued recombinant virus was serially passaged on Vero cells. When Vero cells have just filled the cell flask, inoculate 1 mL of recombinant virus solution that has been repeatedly frozen and thawed three times, and add 4 mL of serum-free DMEM cell maintenance solution, and place it at 37°C, 5% CO 2 Continue to culture in the cell incubator. Harvest the virus when 90% of the cells have cytopathic changes. Place the entire cell flask in a refrigerator at -70°C and freeze-thaw for 3 times, waiting for the next inoculation. In each generation, 1 mL of cytotoxic RNA was extracted, and RT-PCR was used to detect the presence of IBV genome.
[0077] 1.2 Determination of virus titer
[0078] Take the 5th, 10th, 15th, 18th, and 21st generation cytotoxicities of the successfully resc...
Embodiment 3IB
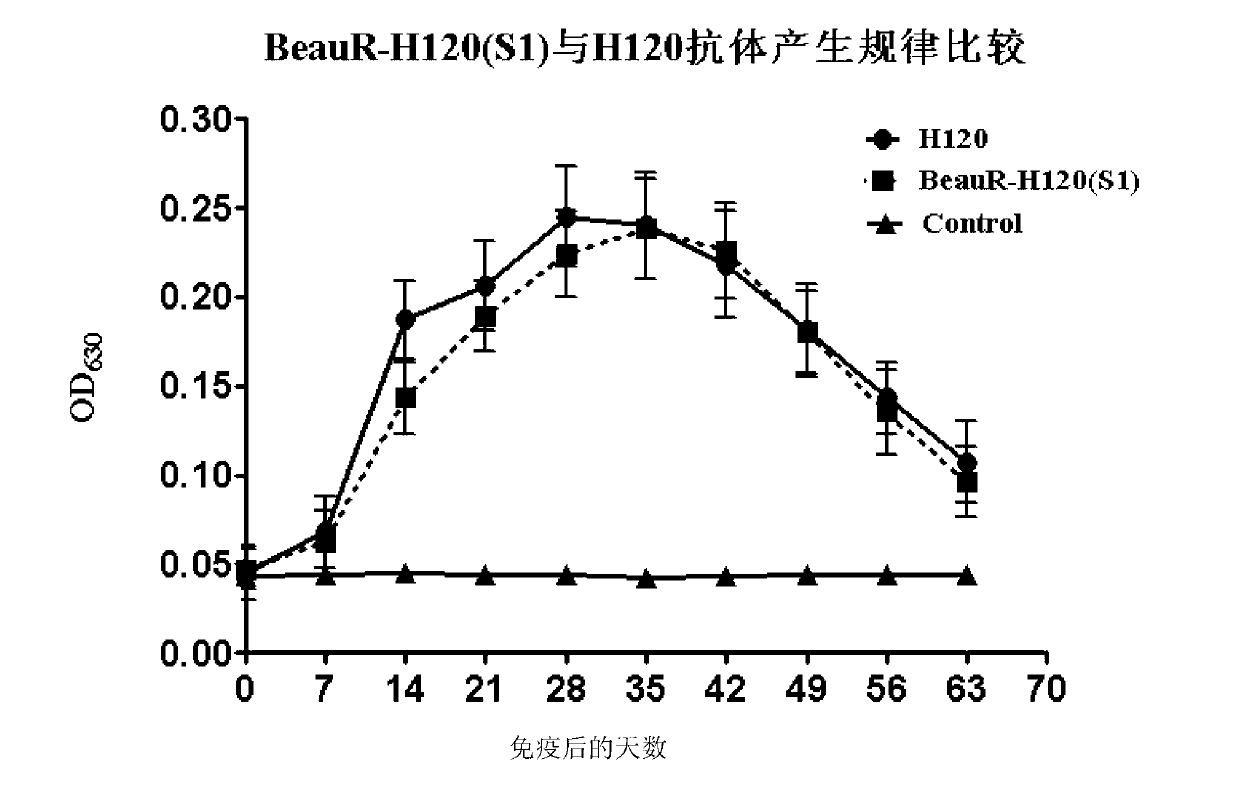
[0100] Example 3 Evaluation of immune efficacy of IBV recombinant virus BeauR-H120 (S1)
[0101] 1 method
[0102] 1.1 Immune protection test of IBV recombinant virus BeauR-H120 (S1)
[0103] Randomly divide 40 1-day-old SPF chicks into two groups A and B (20 birds / group), and rear them in a negative pressure isolator. At the age of 7 days, group A was inoculated with IBV BeauR-H120(S1) cytotoxicity (21st generation) (10 6.25 EID 50 / 0.1mL), each eye drops 0.1mL. Group B was used as the control group, and 0.1 mL of normal Vero cell lysate was injected into each eye. From the day of vaccination, observe and record the morbidity of the vaccinated flocks every day. On 14 and 21 days after immunization, blood was collected from the two flocks and the serum was separated for specific antibody detection. The specific method refers to the instructions of the IBV antibody detection kit from IDEXX, USA. 21 days after immunization, the two groups of chicks were challenged by intranasal dro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com