Use of pyridoheterocyclic ester compounds in manufacture of anti coxsackievirus B3 drugs
A technology of coxsackie virus and heterocyclic ester, which is applied in the directions of active ingredients of heterocyclic compounds, antiviral agents, organic chemistry, etc., can solve the problems of biological activity evaluation and other problems, and achieves low price, simple synthesis process, and easy purchase. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] [Example 1] Evaluate the anti-CVB3 activity of novel pyridine heterocyclic ester compounds
[0021] 1. Test method:
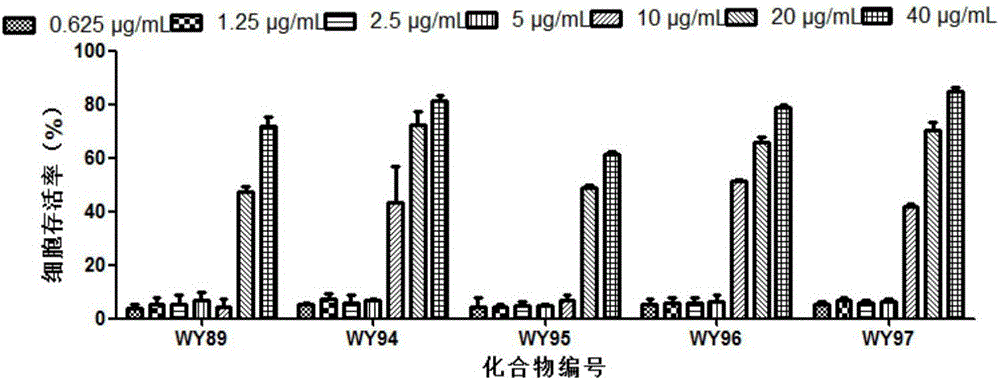
[0022] 1.1 Toxicity of compounds to host Hep-2 cells
[0023] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the monolayer was grown in the incubator, the cell culture solution was discarded, and the cell maintenance solution containing different concentrations of the test compound was added to continue the culture. After 48 hours, the cytotoxicity was visually observed under the microscope and recorded respectively, and the cell survival rate was determined by the MTT method. SPSS 11.5 software calculates the median toxic concentration (Median cyctoxic concentration, CC50) of the drug on the cells. Cell viability=(average OD of drug group 492 Value / average OD of cell control group 492 value) × 100%.
[0024] 1.2 Inhibitory activity of compounds against CVB3
[0025] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 Afte...
Embodiment 2
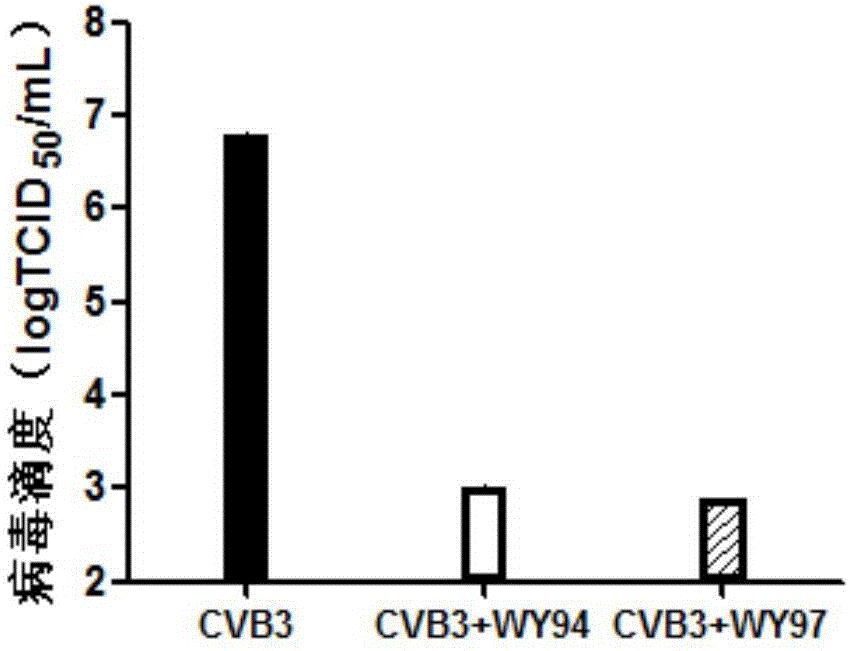
[0034] [Example 2] The inhibitory effect of WY94 and WY97 on the production of CVB3 progeny virus
[0035] 1. Test method
[0036] Hep-2 cells in the logarithmic growth phase were plated in 24-well plates, and 100TCID after the monolayer was overgrown 50 CVB3 infected cells, incubated at 37°C for 1.5h, removed the virus solution, washed three times with PBS, and added cell maintenance solution containing 40 μg / mL WY94 and WY97. After 48 hours, the cells and supernatant were collected and lysed by freezing and thawing three times at -20°C and 37°C. TCID 50 Methods To determine the titer of CVB3 virus.
[0037] 2. Test results
[0038] like image 3 As shown, compared with the virus control group, the Hep-2 cells treated with 40 μg / mL WY94 and WY97 had a significantly decreased virus titer, showing a decrease of about 3.5 log. This shows that the compound has a strong inhibitory effect on the production of CVB3 progeny virus.
Embodiment 3
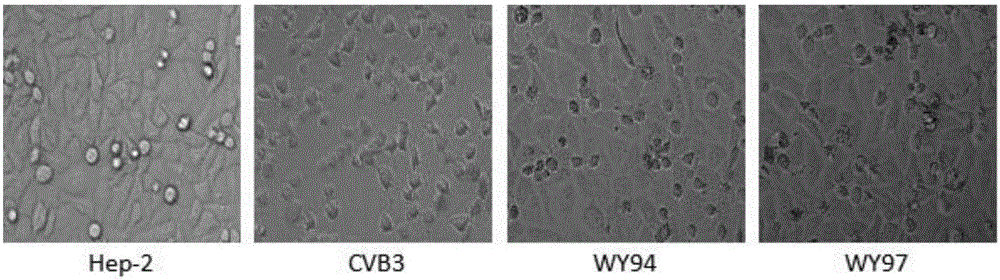
[0039] [Example 3] Inhibitory effect of WY94 and WY97 on Hep-2 cell apoptosis caused by CVB3
[0040] 1. Test method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com