Recombinant protein vaccine, recombinant expression vector containing genes for coding recombinant protein vaccine and application of recombinant protein vaccine
A technology of recombinant protein and expression vector, applied in the biological field, can solve the problems of single EB virus protein, eliminate EB virus, etc., and achieve the effect of preventing EB virus infection, eliminating diseases and tumors, and preventing or treating EB virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
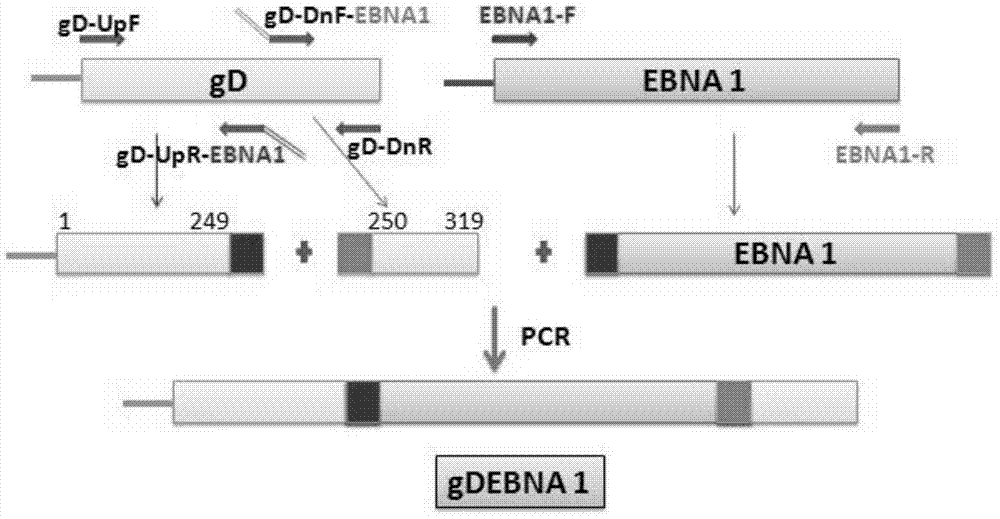
[0059] A method for constructing a recombinant expression vector containing a nucleotide sequence encoding the recombinant protein vaccine according to claims 1 to 5, comprising the following steps:
[0060] (1) Cloning of Epstein-Barr virus nuclear antigen 1 gene sequence
[0061] Using the extracted EBV genome as a template, primers were used: upstream primer EBNA1-F:5'-ATGTCTGACGAGGGGCCAGG-3' (SEQ ID No7) and downstream primer EBAN1-R:5'-CTCCTGCCCTTCCTCACCCT-3' (SEQ ID No.8) EBNA1 was amplified by PCR, and the amplification conditions were:
[0062] The PCR reaction system is (100 μl): 5╳Buffer 20 μl, dNTP mix 8 μl, DNA 6 μl, Taq enzyme 1 μl, primer mixture 4 μl, double distilled water 61 μl;
[0063] The reaction parameters were: 95°C for 2min, 95°C for 15s, 68°C for 15s, 72°C for 3min, a total of 35 cycles, and finally 72°C for 8min.
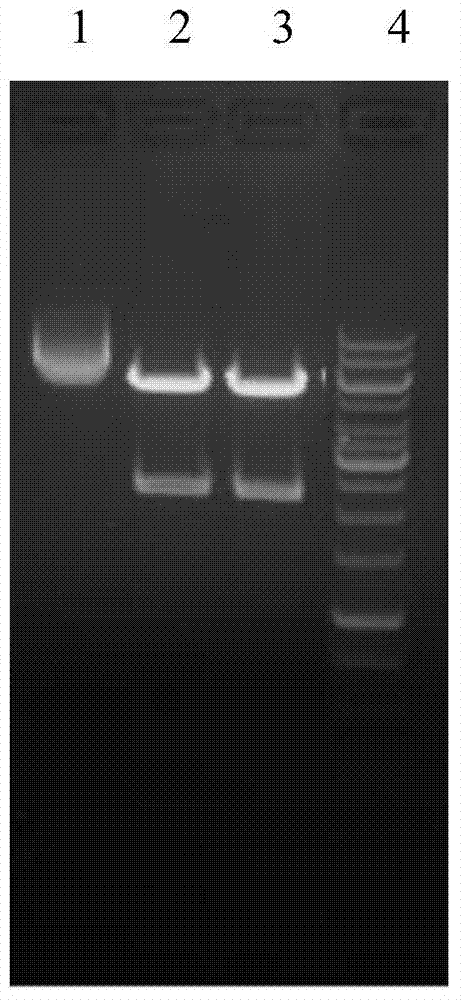
[0064] Then, the PCR product was recovered by agarose gel electrophoresis, and the recovered product was sent to Yingwei Jieji (Shanghai...
Embodiment 2
[0096] This embodiment provides a method for expressing and purifying a recombinant protein vaccine, comprising the following steps:
[0097] (1) Expression of His-Tag tag in Escherichia coli E.Coli
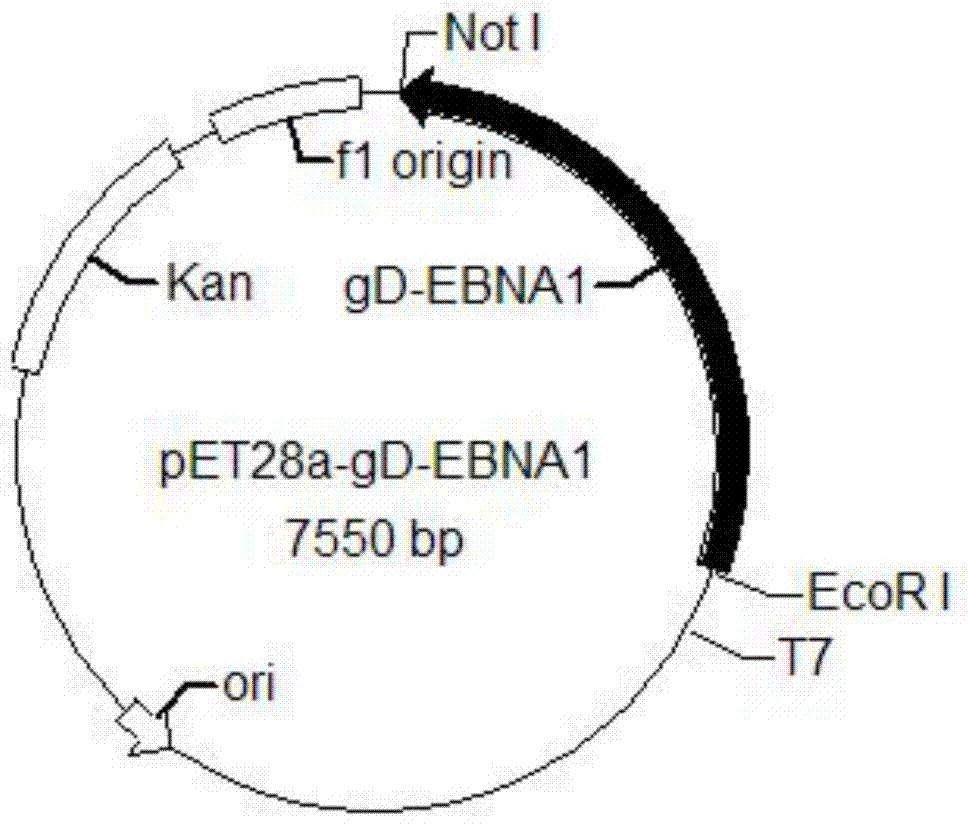
[0098] Transform the pET28a-gD-EBNA1 plasmid into BL21 expression bacteria according to the conventional molecular cloning method in the laboratory to obtain the E.Coli-pET28a-gDEBNA1 strain;
[0099] In 5ml of LB liquid medium containing 50mg / L Kan, inoculate the E.Coli-pET28a-gDEBNA1 bacterial solution at a ratio of 1:50, and culture overnight at 37°C with shaking at 220rpm; the next day, inoculate the cultured bacterial solution overnight until it contains 50mg / L In Kan's new LB liquid medium, culture at 37°C with shaking at 220rpm to OD600=0.6-0.8; add IPTG to each tube to a final concentration of 0.5mmol / L, and induce expression at 28°C; after induction for 2, 4, and 6 hours, respectively Finally, collect the bacterial liquid, collect the bacterial cells by high-speed centr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com