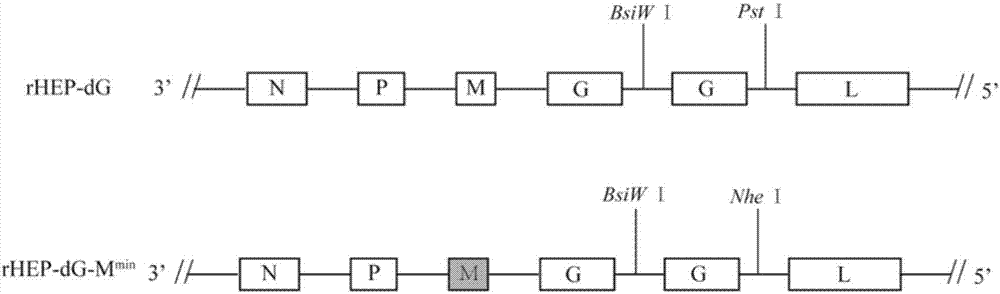
Recombinant rabies virus carrying deoptimized M gene and two G genes
A rabies virus, de-optimized technology, applied in the direction of viruses, viral peptides, antiviral agents, etc., can solve the problems of high production costs, limited production scale, and high prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Optimization of M gene based on mouse codon bias
[0071] By referring to the Codon Usage Database, considering the coordination of nucleic acid sequences before and after optimization, the ratio of CpG and UpA after optimization, and the AU enrichment sequence, the M gene of RABV was optimized (underrepresented) based on mouse-derived codons. , and the reverse bias tropism-optimized M gene was named M-min. The results of M gene deoptimization are shown in Table 1.
[0072] Table 1 Codon preference optimization of RABV M gene
[0073]
[0074] Specifically, the sequence of the original M gene (RABV-M) of RABV is shown in SEQ ID NO.1, and the sequence of the deoptimized M gene (RABV-M-Min) is shown in SEQ ID NO.2.
Embodiment 2
[0075] Example 2 Homologous recombination seamless cloning of M-min fragment and pHEP-3.0 plasmid
[0076] 1. Construction of recombinant plasmid pHEP-3.0-M min
[0077] (1) The M gene was amplified using primers M-min-F / M-min-R (Table 2), and the vector pHEP-3.0 was linearized using primers vHEP-3.0-F / vHEP-3.0-R.
[0078] Table 2 Primers for recombinant rabies virus gene cDNA cloning
[0079]
[0080]
[0081] (2) After the reaction, 5 μL of PCR products were taken for electrophoresis detection on 1.0% agarose gel. M-min amplification PCR obtained a specific DNA electrophoresis band with a size of about 644bp, which was consistent with the test expectation, see figure 2 shown. The vector pHEP-3.0 was linearized to obtain a specific DNA electrophoresis band, which was about 16088bp, which was in line with the experiment expectation, see image 3 shown.
[0082] (3) The PCR amplification product was recovered from the gel DNA, and the recovered M-min fragment was con...
Embodiment 3
[0089] Embodiment 3 utilizes recombinant plasmid pHEP-3.0-M min Construction of recombinant plasmid pHEP-dG-M min
[0090] 1. Using the recombinant plasmid pHEP-3.0-M constructed in Example 2 min Construction of pHEP-dG-M min , see the construction scheme Figure 4 .
[0091] (1) Using the pHEP-3.0 plasmid as a template, use primers RV-G-F / RV-G-R to carry out PCR amplification on the recombinant plasmid pHEP-3.0, and add BsiWI and NheI restriction sites at both ends to obtain rabies virus G gene The complete open reading frame, the amplified length is 1575bp.
[0092] The G gene was amplified using the pHEP-3.0 plasmid as a template, and the reaction system was shown in Table 4 below:
[0093] Table 4 reaction system
[0094] 5×Phusion HF buffer
10.0 μL
dNTPs (10mM)
6μL
Mgcl 2 (10mM)
3.5 μL
pHEP-3.0 plasmid
5.5ng
RV-G-F (10μM)
2.5μL
RV-G-R (10μM)
2.5μL
Phusion polymerase (2U / μL)
0.5μL
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com