Cefpodoxime proxetil pellets and capsules and preparation method thereof
A technology for cefpodoxime axetil and cefpodoxime, which is applied in the field of pharmaceutical preparations, can solve the problems of being difficult to satisfy cefpodoxime axetil taste and stable quality, unfavorable to industrialized production, complicated preparation process, etc., and achieves improved compliance and cost. Low, good taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
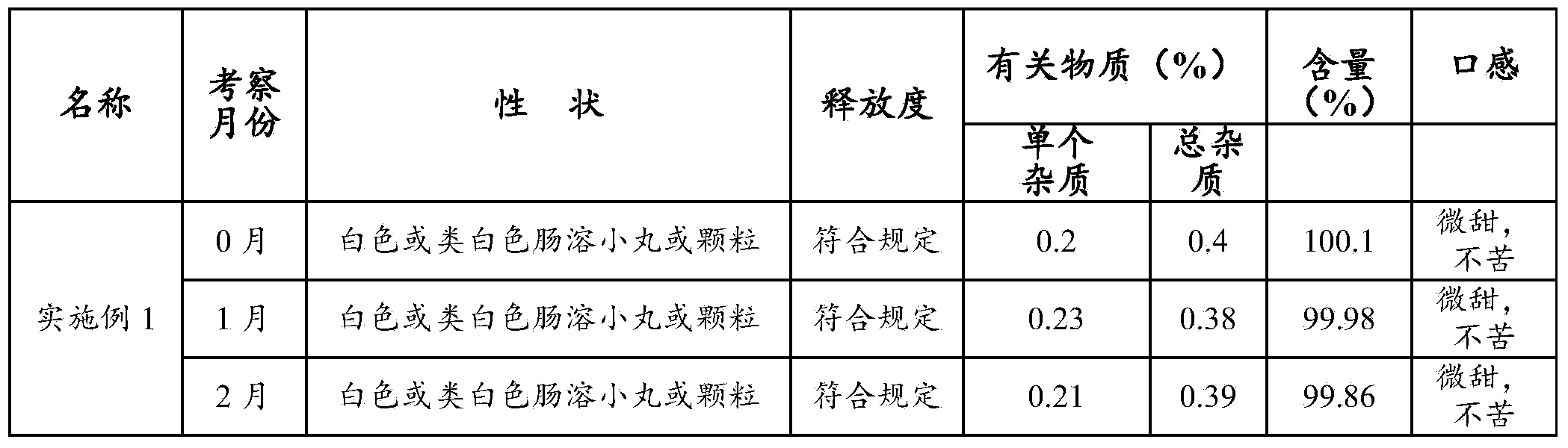
Embodiment 1
[0044] This example is the preferred prescription of the cefpodoxime axetil pellets provided by the present invention, including two parts, the base pill prescription and the protective layer prescription, which are described in detail as follows.
[0045] Cefpodoxime axetil pellets include a base pellet and a protective layer, and the composition is as follows:
[0046] a. Base pill:
[0047]Cefpodoxime Proxetil 60g 60%
[0048] Hydroxypropyl Methyl Cellulose 18g 18%
[0049] Mannitol 8g 8%
[0050] b. Protective coating (i.e. the protective layer):
[0051] Hydroxypropyl Methyl Cellulose 7g 7%
[0052] Diacetylglycerides 3g 3%
[0053] Talc 2g 2%
[0054] Below is the preparation method of the cefpodoxime axetil pellet of the present embodiment, comprises the following steps:
[0055] 1) Preparation of base pellets:
[0056] Dissolve 18g of hydroxypropylmethylcellulose in an appropriate amount of purified water to form a 5% aqueous solution of hydroxypropylmethylcell...
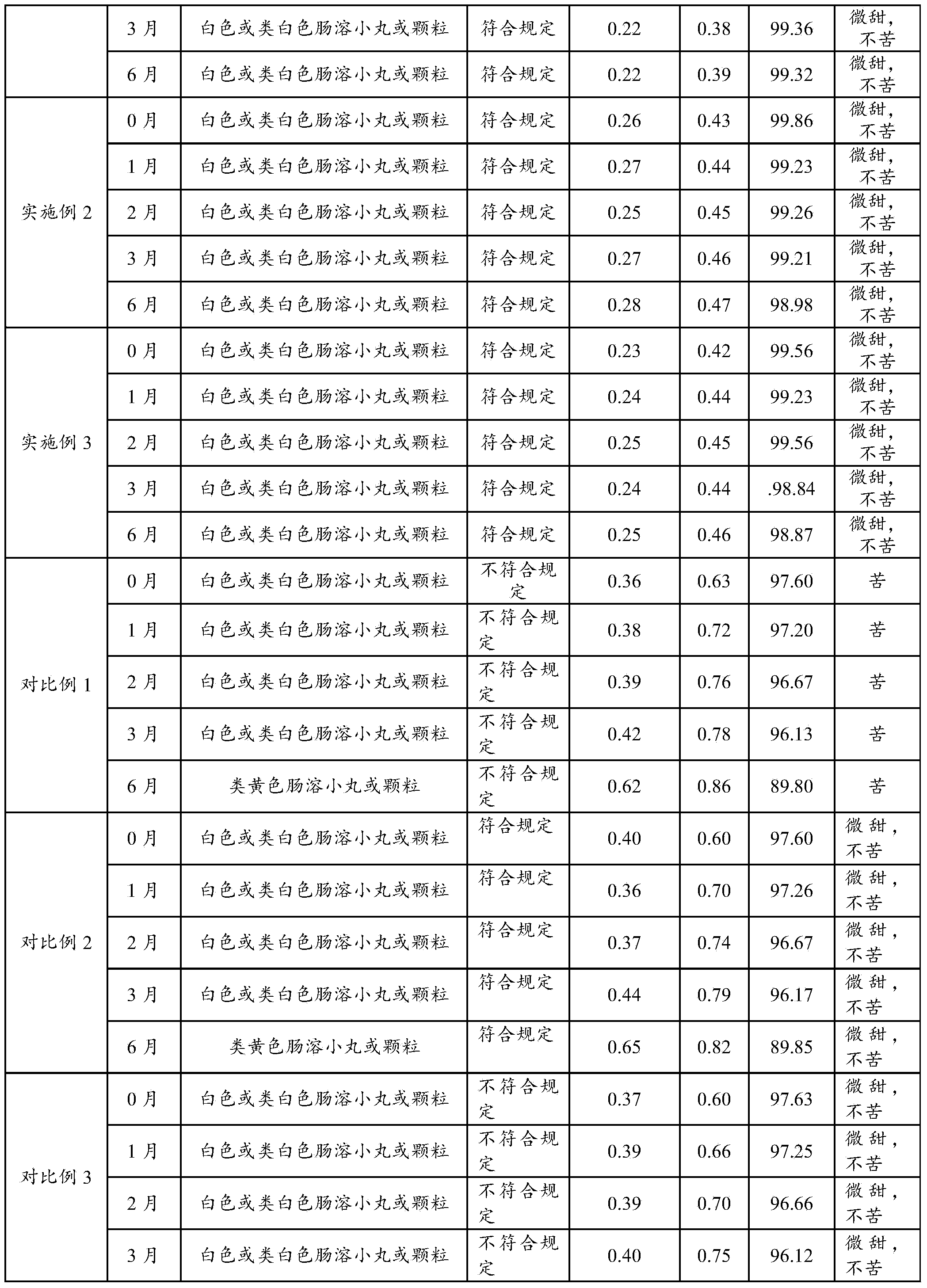
Embodiment 2
[0061] Cefpodoxime axetil pellets comprise base pill and protective layer, preferably following composition:
[0062] a. Base pill:
[0063] Cefpodoxime Proxetil 60g 60%
[0064] Hydroxypropyl Methyl Cellulose 15g 15%
[0065] Mannitol 6g 6%
[0066] b. Protective coating:
[0067] Hydroxypropyl Methyl Cellulose 5g 5%
[0068] Diacetylglycerides 4g 4%
[0069] Talc 2.5g 2.5%
[0070] The present embodiment is the preparation method of cefpodoxime axetil pellet provided by the present invention, comprises the following steps:
[0071] 1) Preparation of base pellets:
[0072] Dissolve 15g of hydroxypropylmethylcellulose in an appropriate amount of purified water to form a 7% aqueous solution of hydroxypropylmethylcellulose, weigh 60g of 80-mesh cefpodoxime axetil, and mix with 5g of mannitol to obtain a mixed powder , take the mixed powder accounting for 15% of the total weight of the mixed powder, place it in the coating pan of the coating granulator (model: BY-600), ad...
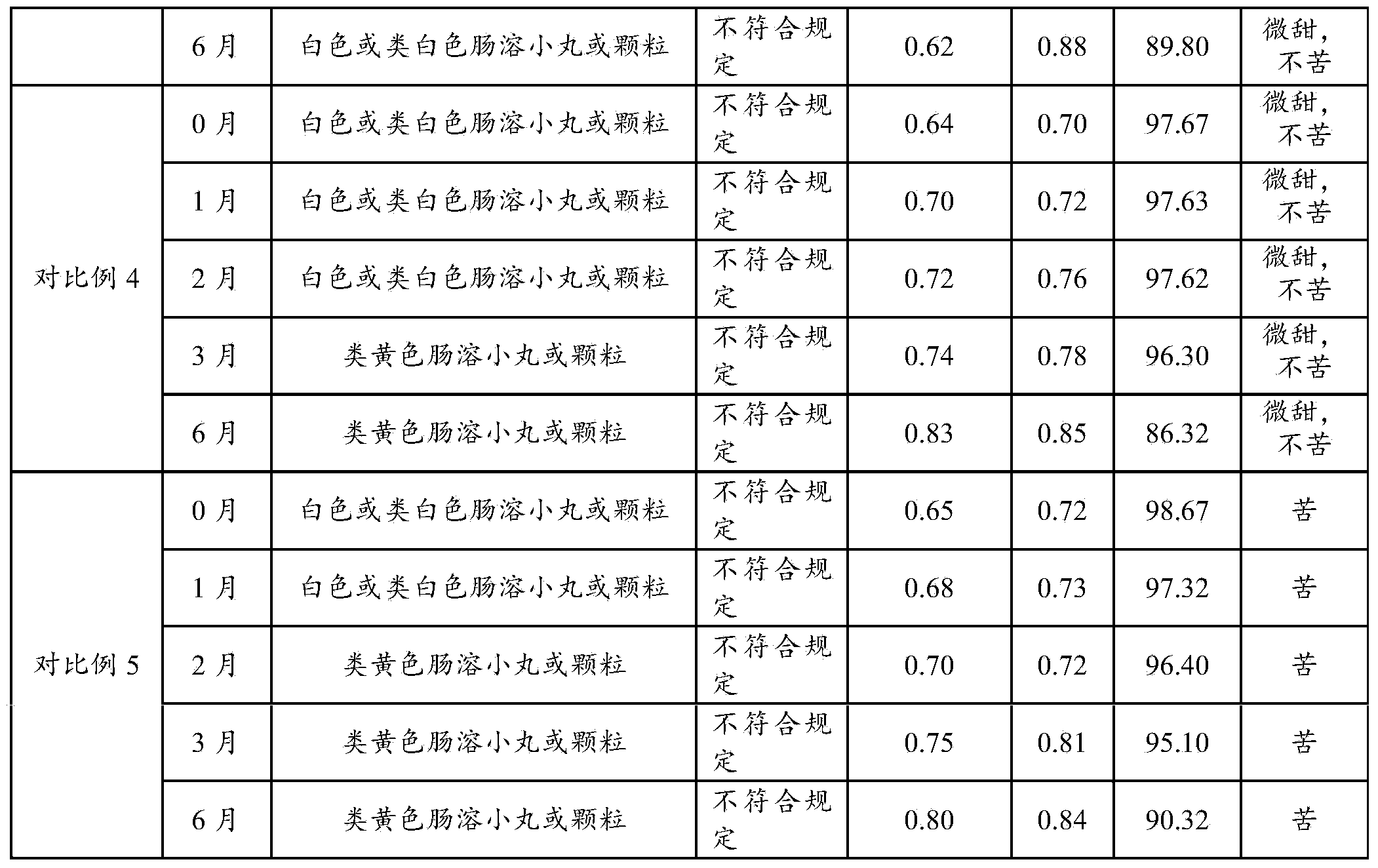
Embodiment 3
[0077] Cefpodoxime axetil pellets comprise base pill and protective layer, preferably following composition:
[0078] a. Base pill:
[0079] Cefpodoxime Proxetil 60g 60%
[0080] Hydroxypropyl Methyl Cellulose 12g 12%
[0081] Mannitol 10g 10%
[0082] b. Protective coating:
[0083] Hydroxypropyl Methyl Cellulose 8g 8%
[0084] Diacetylglycerides 3g 3%
[0085] Talc 2g 2%
[0086] The present embodiment is the preparation method of cefpodoxime axetil pellet provided by the present invention, comprises the following steps:
[0087] 1) Preparation of base pellets:
[0088] Dissolve 12g of hydroxypropylmethylcellulose in an appropriate amount of purified water to form a 6% aqueous solution of hydroxypropylmethylcellulose, weigh 60g of 80-mesh cefpodoxime axetil, and mix with 10g of mannitol to obtain a mixed powder , take the mixed powder accounting for 15% of the total weight of the mixed powder, place it in the coating pan of the coating granulator (model: BY-600), adj...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com