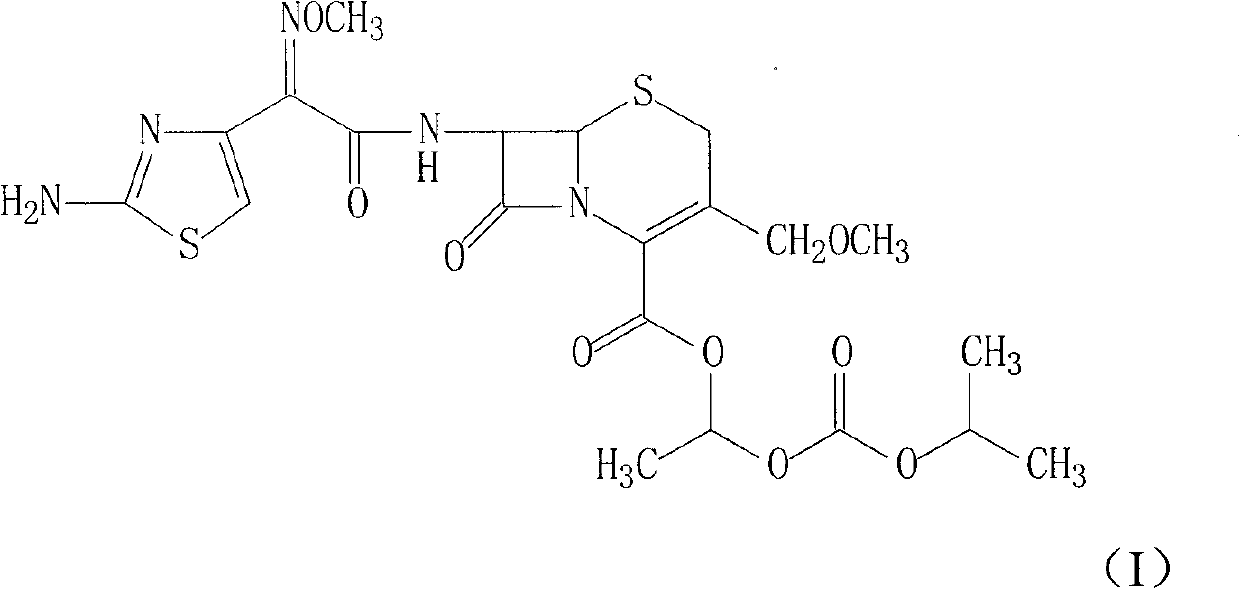
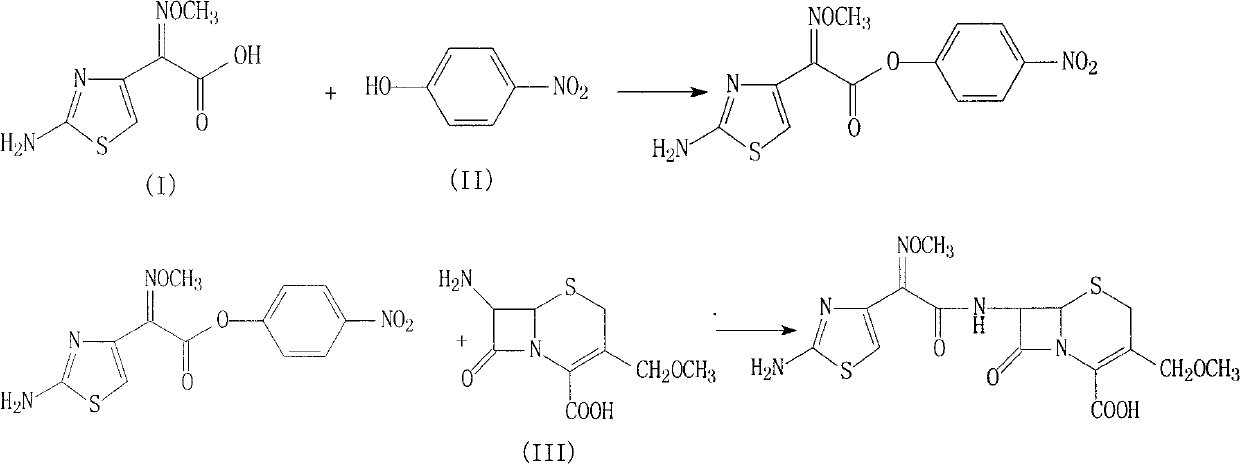
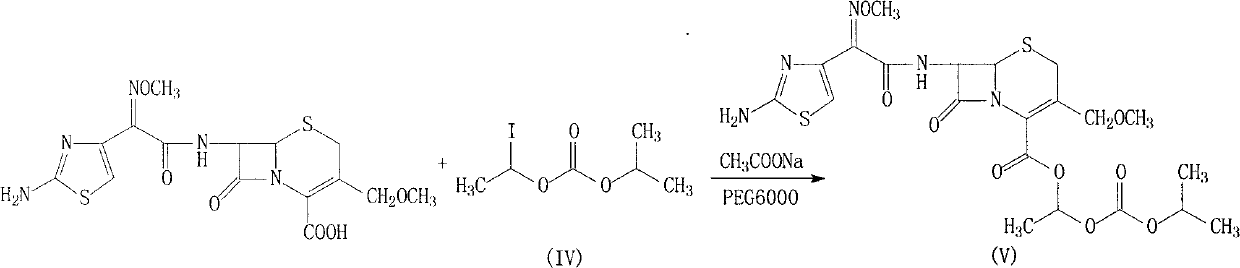
Cefpodoxime proxetil compound of new route
A technology for cefpodoxime axetil and cefpodoxime acid, which is applied in the field of medicine, can solve the problems of high isomer content, low product purity, slow reaction and the like, and achieves the effects of high purity, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 cefpodoxime axetil
[0031] (1) Add 201g of (Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid and 110ml triethylamine into 500ml dichloromethane, cool to 10°C, stir, Add 167g of p-nitrophenol, stir and react at this temperature for 1 hour, then add the solution formed by 244g of 7-AMCA, 200ml of triethylamine and 500ml of dichloromethane, react at 10°C for 3 hours, stir, add 3L of water, Regulate the pH with 10% hydrochloric acid to be 5, separate layers, wash the water phase with 500ml dichloromethane once, separate the layers, adjust the pH of the system with 10% hydrochloric acid to be 2.5, stir, precipitate solids, filter, and use Washed with 100ml of acetone and dried under vacuum at 40°C to obtain 397g of the product with a yield of 92.8%.
[0032] (2) Add 397g of cefpodoxamic acid to 2000ml of N,N-dimethylformamide, stir to dissolve, add 91.3g of sodium acetate and 10ml of water, stir at room temperature for 30min, cool to 5°C...
Embodiment 2
[0033] The preparation of embodiment 2 cefpodoxime axetil
[0034] (1) Add 201g of (Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid and 110ml triethylamine into 500ml dichloromethane, cool to 5°C, stir, Add 167g of p-nitrophenol, stir and react at this temperature for 1 hour, then add the solution formed by 244g of 7-AMCA, 200ml of triethylamine and 500ml of dichloromethane, react at 5°C for 3 hours, stir, add 3L of water, Regulate the pH with 5% hydrochloric acid to be 6, separate layers, wash the water phase with 500ml dichloromethane once, separate the layers, adjust the pH of the system with 5% hydrochloric acid to be 3, stir, separate out solids, filter, use Washed with 100ml of acetone and dried under vacuum at 50°C to obtain 402g of the product with a yield of 94%.
[0035](2) Add 402g of cefpodoxamic acid into 2000ml of acetonitrile, stir to dissolve, add 92.5g of sodium acetate and 20ml of water, stir at room temperature for 30min, cool to 6°C, add 38g of PEG600...
Embodiment 3
[0036] The preparation of embodiment 3 cefpodoxime axetil
[0037] (1) Add 201g of (Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid and 110ml of triethylamine into 500ml of acetonitrile, cool to 8°C, stir, and add 167g For p-nitrophenol, stir and react at this temperature for 1 hour, then add a solution formed by 244g of 7-AMCA, 200ml of triethylamine and 500ml of acetonitrile, react at 8°C for 3 hours, stir, add 3L of water, and use 15% Regulate the pH with hydrochloric acid to be 5.5, separate layers, wash the aqueous phase with 500ml acetonitrile once, separate the layers, adjust the pH of the system to 2.8 with 15% hydrochloric acid for the aqueous phase, stir, precipitate solids, filter, wash with 100ml acetone, and wash at 45 Vacuum-dried at °C to obtain 399.6 g of the product, with a yield of 93.5%.
[0038] (2) Add 399.6g of cefpodoxamic acid into 2000ml of tetrahydrofuran, stir to dissolve, add 92g of sodium acetate and 20ml of water, stir at room temperature fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com