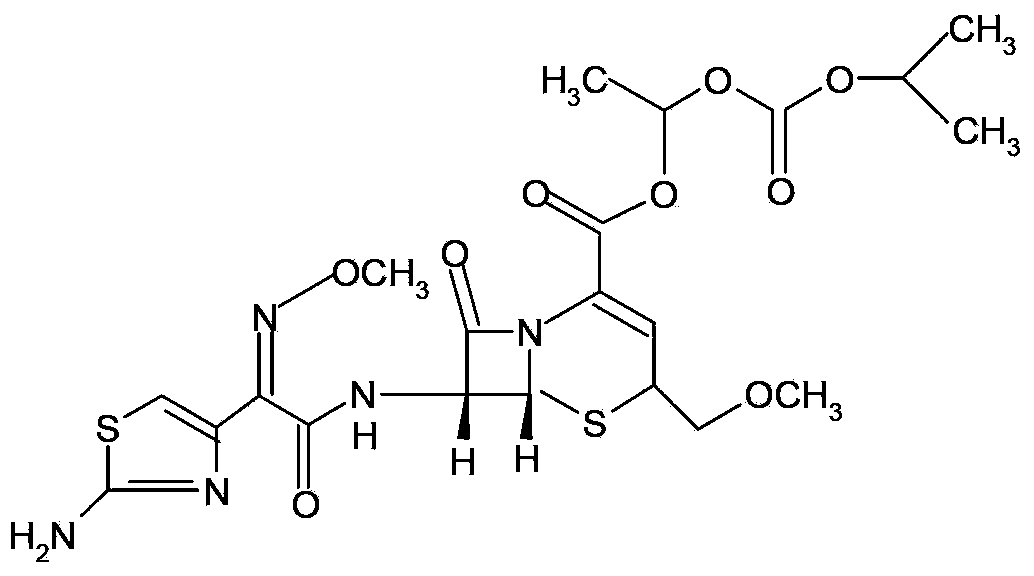
Cefpodoxime proxetil composition dry suspension and preparation method thereof
A technology of cefpodoxime axetil and dry suspension, which is applied in the field of medicine, can solve problems such as unfavorable swallowing, poor dissolution rate, and poor compliance of patients to take, and achieve drug agglomeration, high dissolution rate, stability and safety small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 cefpodoxime axetil composition dry suspension
[0035]
[0036] Take cefpodoxime axetil and sucrose to pass through a 100-mesh sieve, add mannitol and mix, add starch slurry to make soft material, pass through a 40-mesh sieve to granulate, then dry at 45-50°C for 25-30 minutes, and pass through a 40-mesh sieve Serve whole grains.
Embodiment 2
[0037] The preparation of embodiment 2 cefpodoxime axetil composition dry suspension
[0038]
[0039] Take cefpodoxime axetil and sucrose to pass through a 100-mesh sieve, add mannitol and mix, add starch slurry to make soft material, pass through a 40-mesh sieve to granulate, then dry at 45-50°C for 25-30 minutes, and pass through a 40-mesh sieve Serve whole grains.
Embodiment 3
[0040] The preparation of embodiment 3 cefpodoxime axetil composition dry suspension
[0041]
[0042] Take cefpodoxime axetil and sucrose to pass through a 100-mesh sieve, add mannitol and mix, add starch slurry to make soft material, pass through a 40-mesh sieve to granulate, then dry at 45-50°C for 25-30 minutes, and pass through a 40-mesh sieve Serve whole grains.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com