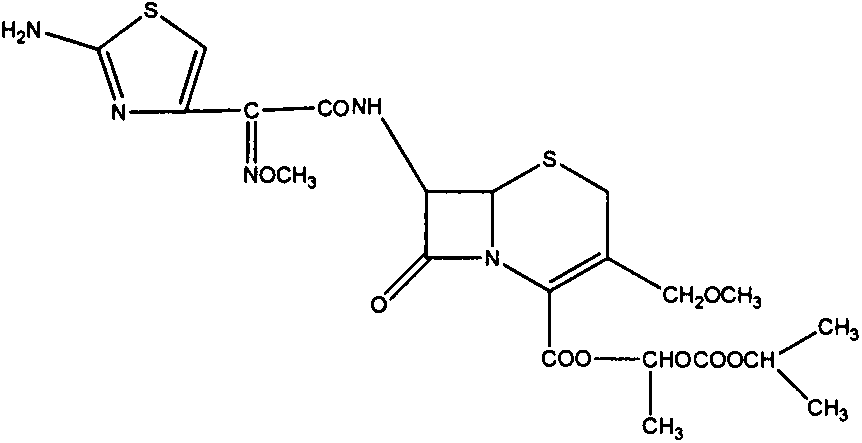
Cefpodoxime proxetil granules and preparation method thereof
A technology of cefpodoxime axetil and granules, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of peculiar smell, limited use, etc. , Low product cost, good taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
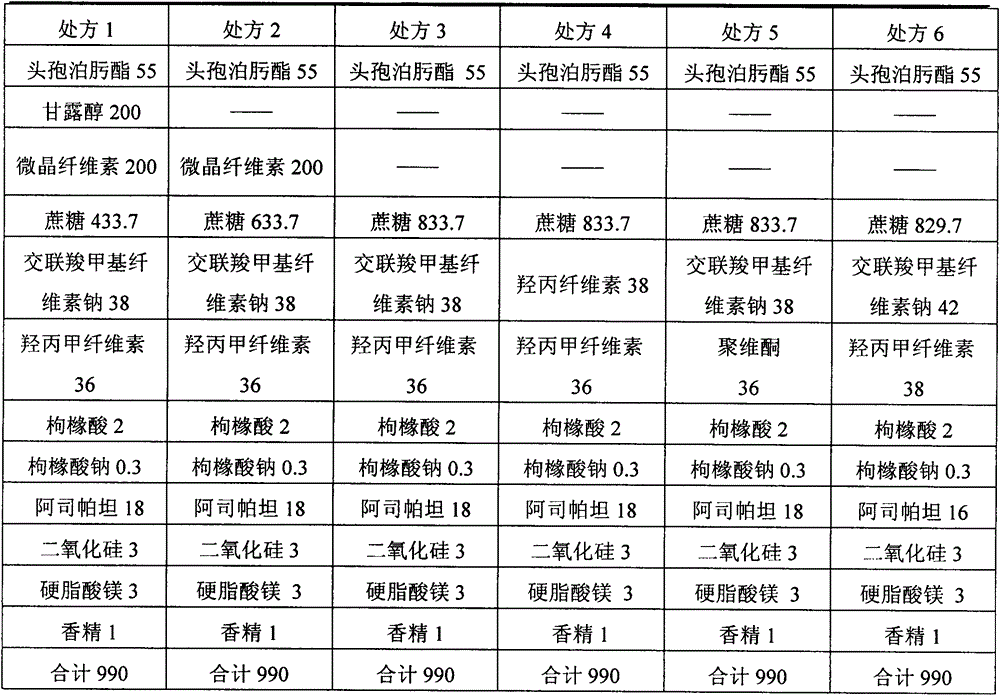
[0110] Product Prescription:
[0111] Name of raw material
40,000 bags (kg)
cefpodoxime axetil
2.080
32.912
1.640
1.640
0.080
[0112] sodium citrate
0.008
0.720
0.240
silica
0.240
fragrance
0.040
gross weight
39.600
[0113] Preparation Process:
[0114] (1) Pretreatment of raw and auxiliary materials:
[0115] Sucrose is crushed through a 60-mesh sieve, croscarmellose sodium, hydroxypropyl methylcellulose, aspartame, magnesium stearate, essence, citric acid, and sodium citrate are mixed and passed through a 60-mesh sieve. Silicon oxide is passed through a 120-mesh sieve for subsequent use; the cefpodoxime axetil raw material is mixed with 2 times the pulverized sucrose and passed through a 60-mesh sieve for subsequent use;
[0116] (2) mixin...
Embodiment 2
[0123] Product Prescription:
[0124] Name of raw material
60,000 bags (kg)
cefpodoxime axetil
3.120
49.368
2.460
2.460
0.120
sodium citrate
0.012
1.080
0.360
silica
0.360
fragrance
0.060
gross weight
59.400
[0125] Preparation Process:
[0126] (1) Pretreatment of raw and auxiliary materials:
[0127] Sucrose is crushed through a 60-mesh sieve, croscarmellose sodium, hydroxypropyl methylcellulose, aspartame, magnesium stearate, essence, citric acid, and sodium citrate are mixed and passed through a 60-mesh sieve. Silicon oxide is passed through a 120-mesh sieve for subsequent use; the cefpodoxime axetil raw material is mixed with 2 times the pulverized sucrose and passed through a 60-mesh sieve for subsequent use;
[0128] (2) mixing:
[0129] The mi...
Embodiment 3
[0135] Product Prescription:
[0136] Name of raw material
40,000 bags (kg)
cefpodoxime axetil
2.080
sucrose
33.232
1.520
1.520
citric acid
0.080
0.008
aspartame
0.800
Magnesium stearate
0.160
silica
0.160
fragrance
0.040
gross weight
39.600
[0137] Preparation Process:
[0138] (1) Pretreatment of raw and auxiliary materials:
[0139] Sucrose is crushed through a 60-mesh sieve, croscarmellose sodium, hydroxypropyl methylcellulose, aspartame, magnesium stearate, essence, citric acid, and sodium citrate are mixed and passed through a 60-mesh sieve. Silicon oxide is passed through a 120-mesh sieve for subsequent use; the cefpodoxime axetil raw material is mixed with 2 times the pulverized sucrose and passed through a 60-mesh sieve for subsequent use;
[0140] (2) mixing:
[0141] The mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com