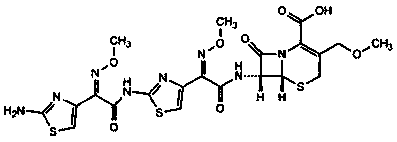
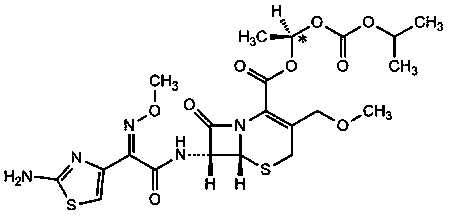
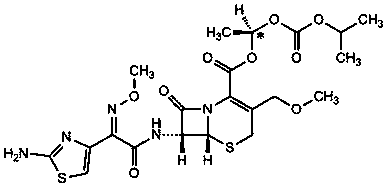
Cefpodoxime proxetil impurity, preparation method thereof and application of cefpodoxime proxetil impurity
A technology of cefpodoxime axetil and impurities, applied in the field of drug preparation, to achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the present embodiment provides the concrete preparation method of formula I cefpodoxime axetil impurity, and the steps are as follows:
[0047] Add 10.0g of formula II compound and 16.4g of 2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetic acid thiobenzothiazolyl ester (AE active ester) to 20ml tetrahydrofuran and 100ml dihydrofuran In methyl chloride, the temperature was controlled at 20°C to 25°C, 4.7g of triethylamine was added, and the reaction was stirred for 5h.
[0048] Add the reaction solution into 100ml of ice water, extract and separate the layers, take the water phase and adjust the pH to 2.5-3.0 with 2mol / L hydrochloric acid, filter, and dry the filter cake under reduced pressure at 40°C to obtain 8.6g of a yellow solid (compound of formula IV), with a purity of 81.5 %.
[0049] Dissolve 8.0g of the compound of formula IV in 50ml of N,N-dimethylacetamide under stirring, control the temperature at -5°C to -10°C, slowly add 4.06g of 1-iodoethyl i...
Embodiment 2
[0051] Embodiment 2, this embodiment provides the specific preparation method of formula I cefpodoxime axetil impurity, the steps are as follows:
[0052] 10.0g formula VI compound and 43.7g 2-[2-[2-(2-amino-4-thiazolyl)-2-(methoxyimino)]acetamido-4-thiazolyl]-2-( Methoxyimino) thiobenzothiazolyl acetate was added to 20ml of tetrahydrofuran and 100ml of dichloromethane, the temperature was controlled at 20°C to 25°C, 8.3g of triethylamine was added, and the reaction was stirred for 5h.
[0053] Add the above reaction solution into 100ml of ice water, extract and separate the layers, take the water phase and adjust the pH to 2.5-3.0 with 2mol / L hydrochloric acid, filter, and dry the filter cake under reduced pressure at 40°C to obtain 16.2g of a yellow solid (compound of formula IV), with a purity of 83.1%.
[0054] Dissolve 8.0g of the compound of formula IV in 50ml of N,N-dimethylacetamide under stirring, control the temperature at -5°C to -10°C, slowly add 4.06g of 1-iodoet...
Embodiment 3
[0056] Embodiment 3, this embodiment provides the specific preparation method of formula I cefpodoxime axetil impurity, and the steps are as follows:
[0057] Add 10.0g of the compound of formula VIII and 12.5g of AE active ester into 100ml of dichloromethane, control the temperature at 25°C to 30°C, add 3.6g of triethylamine, and stir for 8h.
[0058] The reaction solution was added to 100ml of ice water, and the layers were extracted. Take the organic phase and add 50ml of water, and use hydrochloric acid to adjust the pH to 0.5-1.0. After standing for stratification, take the water phase, adjust the pH to neutral with 5% sodium bicarbonate solution, filter, and dry the filter cake under reduced pressure at 40°C to obtain a yellow solid. (Cefpodoxime axetil impurity) 2.6g, purity 75.5%. MS showed that the component m / z: [M+H]+ was 741, and the corresponding molecular weight was 740, which was consistent with the HPLC-MS data of the impurity in the chromatogram of the cefpod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com