Cefpodoxime proxetil suspension composition and preparation thereof
A technology of cefpodoxime axetil and dry suspension, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, powder delivery, etc., can solve problems such as inability to effectively mask bitterness, reduce patients' compliance with clinical medication, etc. Medication compliance, ease of acceptance, and bitterness-improving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
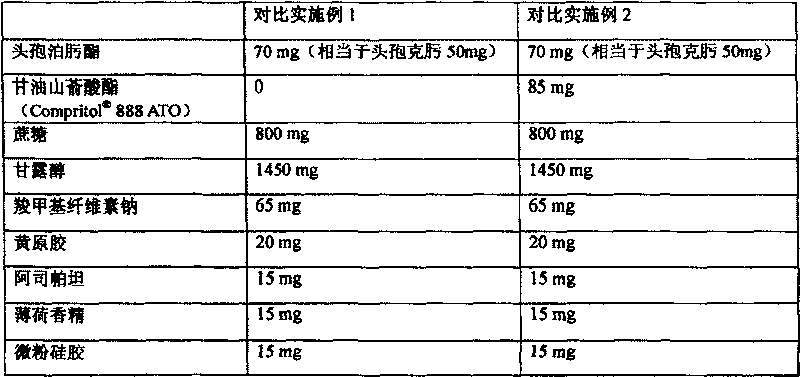
[0061] Formulation of dry suspension 1
[0062] Composition of preparation prescription Content of components in each package of dry suspension (mg)
[0063] Cefpodoxime axetil 70mg (equivalent to cefpodoxime 50mg)
[0064] Glyceryl behenate ( 888ATO) 85mg
[0065] Mannitol 1450mg
[0066] Sucrose 800mg
[0067] Sodium carboxymethylcellulose 65mg
[0068] Xanthan Gum 20mg
[0069] Peppermint Flavor 15mg
[0070] Aspartame 15mg
[0071] Micronized silica gel 15mg
[0072] Preparation method: sieve cefpodoxime axetil (Aurobindo Pharma Ltd. India Arabindu company) and 888ATO (French Gattefosse S.A), mix them evenly. The mixture was placed in a constant temperature oven at 80-85°C and heated for 10-15 minutes to make cefpodoxime axetil and 888ATO co-melted. The molten mixture was then cooled to room temperature, followed by crushing and sieving through a 60-mesh sieve. Add sucrose (Guangxi Liuxing Sugar Industry Co., Ltd.), mannitol (Qingdao Mingyue Seaweed Group C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com