Cefpodoxime proxetil tablet and production process thereof
A technology of cefpodoxime axetil and tablets, applied in the field of cefpodoxime axetil tablets and its production process, can solve the problems of unfavorable industrial production, low yield of tablets, complicated preparation process, etc., achieve low cost, reduce preparation steps, process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
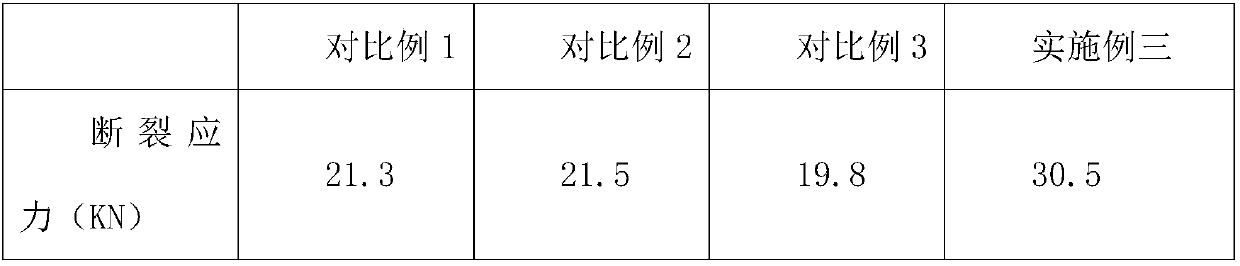
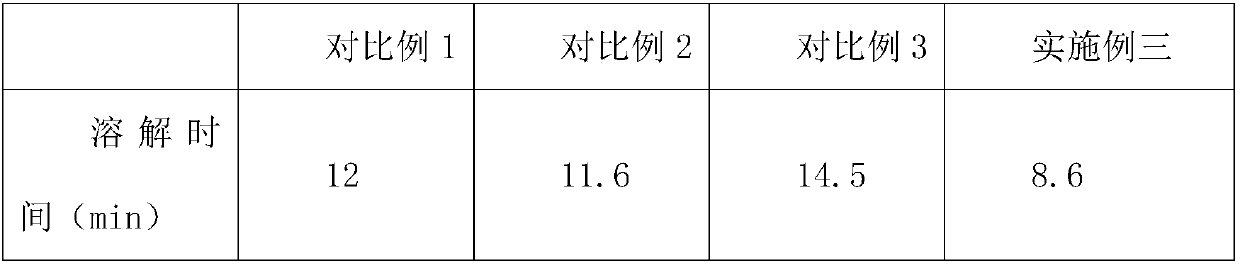
[0020] A cefpodoxime axetil tablet, comprising the following raw materials in parts by weight: 30 parts of cefpodoxime axetil crude product, 10 parts of organic solvent A, 5 parts of organic weak alkaline salt solution, 10 parts of gac, 1-iodoethyl isopropyl 5 parts of carbonate, 6 parts of organic solvent B, 2 parts of filler, 2 parts of disintegrant, 6 parts of binder aqueous solution, 3 parts of EPO, 1 part of flavoring agent, 2 parts of sodium alginate, 1 part of isomalt.
[0021] The organic solvent A is a mixture of methanol, chloroform and tetrahydrofuran in a weight ratio of 1:2:4, and the organic solvent B is N,N-dimethylacetamide.
[0022] The filler is microcrystalline cellulose, the disintegrant is sodium carboxymethyl starch, and the flavoring agent is artificial chicken essence.
[0023] A kind of production technology of described cefpodoxime axetil tablet, comprises the following steps:
[0024] The preparation of S1 cefpodoxime axetil: dissolve cefpodoxime ...
Embodiment 2
[0031] A cefpodoxime axetil tablet comprises the following raw materials in parts by weight: 35 parts of cefpodoxime axetil crude product, 12 parts of organic solvent A, 8 parts of organic weak alkaline salt solution, 13 parts of gac, 1-iodoethyl isopropyl 7 parts of carbonate, 8 parts of organic solvent B, 4 parts of filler, 3 parts of disintegrant, 8 parts of binder aqueous solution, 4 parts of EPO, 2 parts of flavoring agent, 3 parts of sodium alginate, 2 parts of isomalt.
[0032] The organic solvent A is a mixture of methanol, chloroform and tetrahydrofuran in a weight ratio of 1:1:4, and the organic solvent B is N,N-dimethylacetamide.
[0033] The filler is anhydrous lactose, the disintegrant is sodium carboxymethyl starch, and the flavoring agent is artificial beef essence.
[0034] A kind of production technology of described cefpodoxime axetil tablet, comprises the following steps:
[0035] The preparation of S1 cefpodoxime axetil: dissolve cefpodoxime axetil crude...
Embodiment 3
[0042] A cefpodoxime axetil tablet comprises the following raw materials in parts by weight: 40 parts of cefpodoxime axetil crude product, 15 parts of organic solvent A, 10 parts of organic weak alkaline salt solution, 15 parts of gac, 1-iodoethyl isopropyl 8 parts of carbonate, 9 parts of organic solvent B, 5 parts of filler, 4 parts of disintegrant, 9 parts of binder aqueous solution, 5 parts of EPO, 4 parts of flavoring agent, 5 parts of sodium alginate, 3 parts of isomalt.
[0043] The organic solvent A is a mixture of methanol, chloroform and tetrahydrofuran in a weight ratio of 1:1:4, and the organic solvent B is N,N-dimethylacetamide.
[0044] The filler is maltodextrin, the disintegrant is sodium carboxymethyl starch, and the corrective agent is yeast powder.
[0045] A kind of production technology of described cefpodoxime axetil tablet, comprises the following steps:
[0046] The preparation of S1 cefpodoxime axetil: dissolve cefpodoxime axetil crude product in or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com