Cefpodoxime proxetil compound as well as preparation method and medicinal composition thereof
A technology of cefpodoxime axetil and a compound, applied in the field of medicine, can solve the problems of poor powder fluidity and poor stability, and achieve the effects of good dispersibility and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
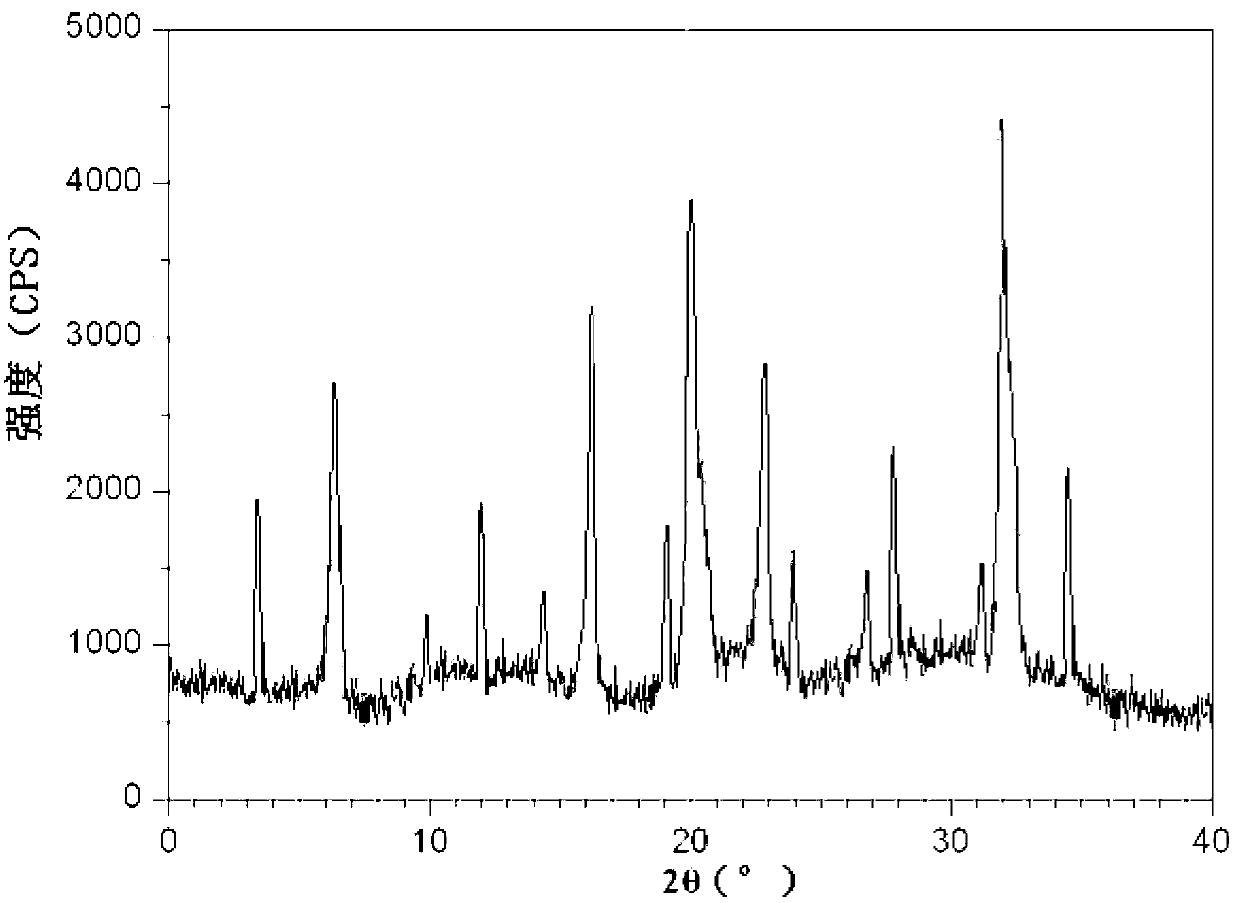
[0049] [embodiment 1] the preparation of cefpodoxime axetil compound
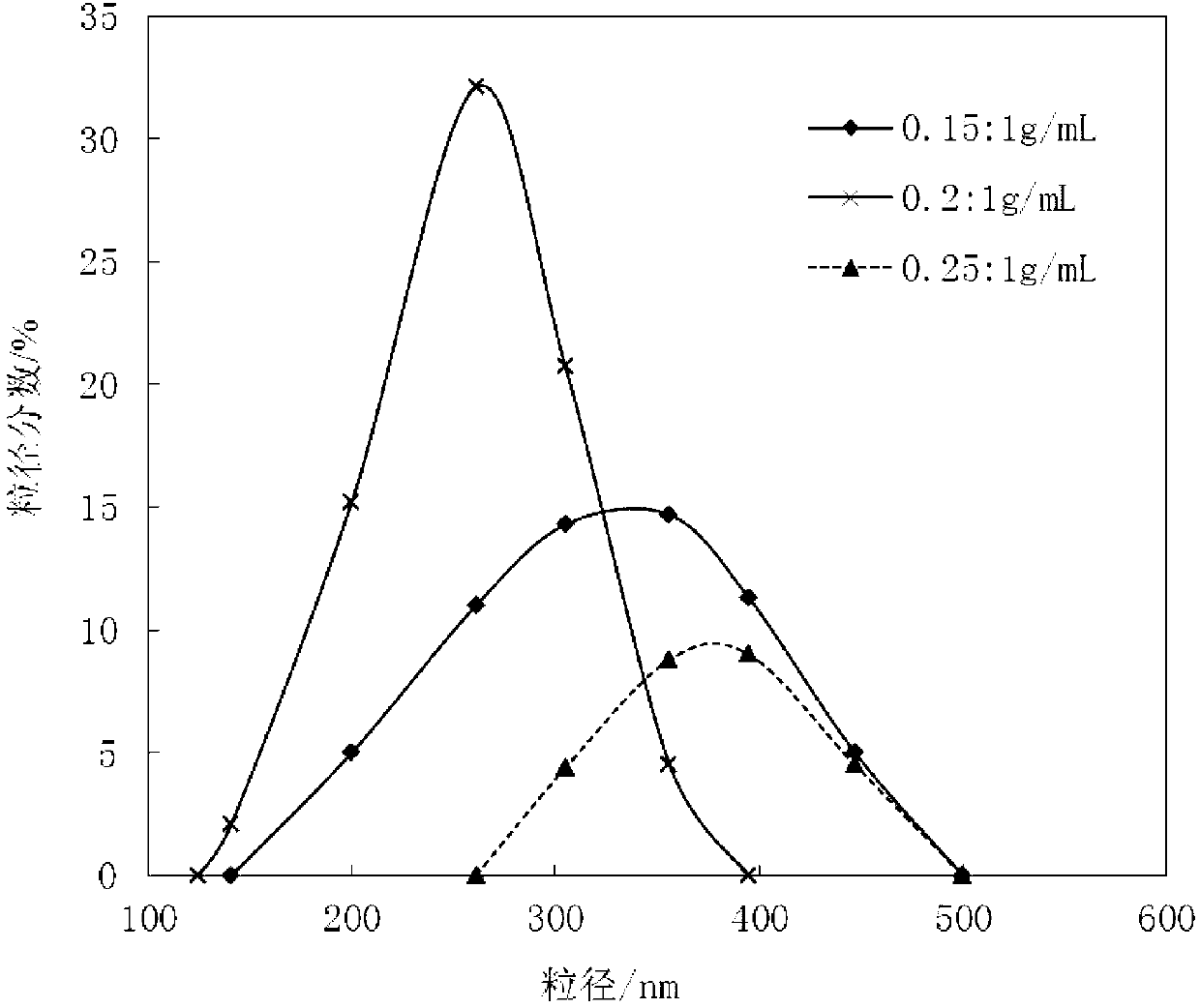
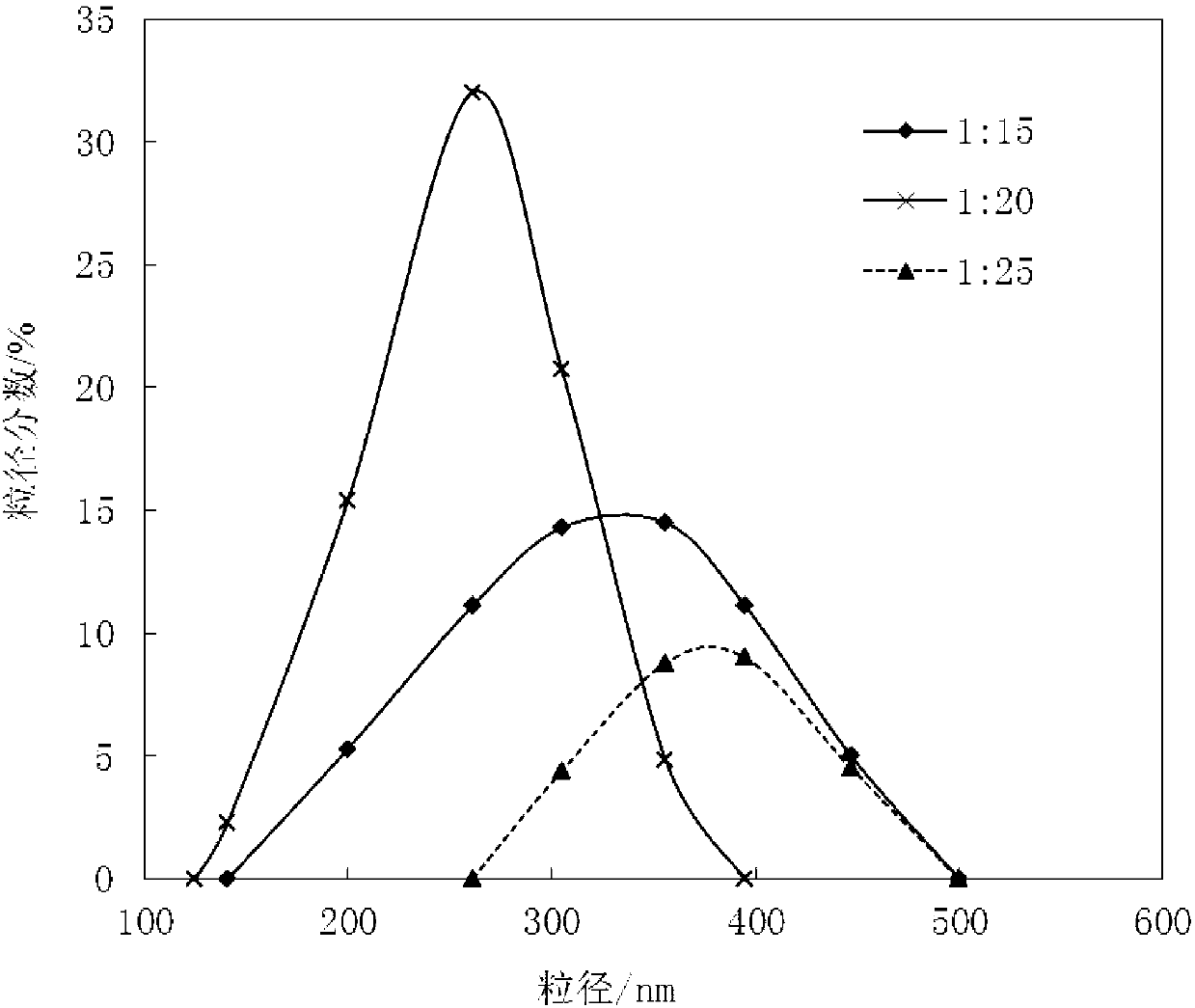
[0050] 1) Add 2.1kg of cefpodoxime axetil raw material into 10L of mixed solvent prepared from methanol and dimethylformamide (the volume ratio of methanol to dimethylformamide is 3:1), stir to dissolve For the medicinal liquid, add active carbon with a total volume of 0.1% g / ml of the medicinal liquid to decolorize, filter, and the filtrate is for subsequent use;
[0051] 2) At room temperature, add the filtrate obtained in step 1) to 200L of water at a stirring speed of 900r / min, cool down to 3°C after adding the filtrate, and continue stirring for 10min after obtaining crystals; filter and wash the filter cake with methanol , and dried under reduced pressure to obtain light yellow crystalline powder, which is the cefpodoxime axetil compound. IR (VBr) cm -1 1780 (beta-lactam C=O), 1680 (amide C=O), 1 HNMR1.31 (6H, d, CH (CH 3 ) 2 ), 1.56 (3H,d,CHCH 3 ) 3.30 (3H, S, OCH 3 ) 3.52 (2H, brS, 2-CH 2 ),...
Embodiment 2-8
[0055]
[0056] The X-ray powder diffraction spectrum obtained by measuring the cefpodoxime axetil compound prepared in Examples 2-8 using Cu-Kα rays is consistent with Example 1.
preparation Embodiment 1
[0057] [Preparation Example 1] Cefpodoxime Proxetil Tablet
[0058] In this example, the cefpodoxime axetil compound prepared in Example 1 of the present invention is used to prepare a pharmaceutical composition in tablet form. Oral formulations in the form of tablets are prepared using dry granulation as a processing technique. The pharmaceutical composition is listed in Table 2.
[0059] Table 2
[0060] Element
[0061] Preparation method: Mix cefpodoxime axetil of Example 1 with sodium carboxymethylcellulose and sodium lauryl sulfate, and pass through a sieve (British Standard Sieve (BBS) 25; 600 μm). Pass the mixture through a pulverizer mill fitted with a 1.0 mm screen and impact forward at high speed. Lactose and hydroxypropylcellulose were passed through a 600 μm mesh sieve and mixed with the milled mixture in a non-shear mixer (octagonal mixer). The resulting mixture was dried into granules by extrusion. The extrudate was passed through a 710 μm mesh (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com