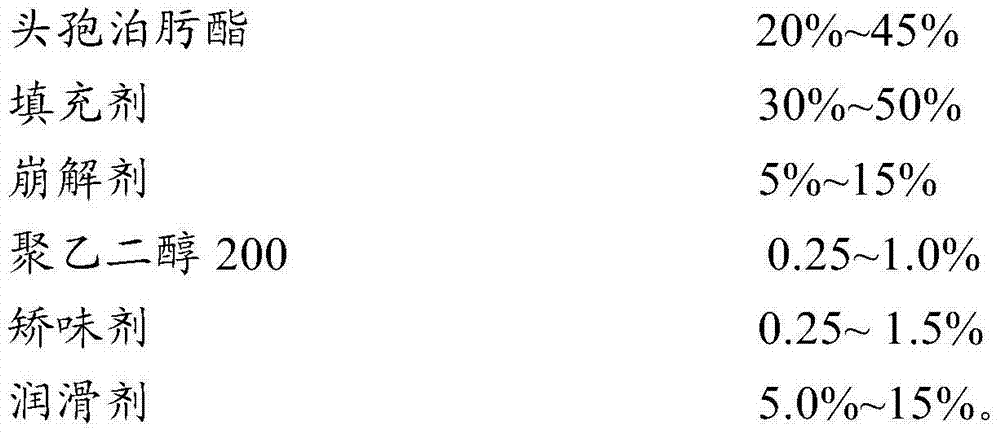Cefpodoxime proxetil dispersible tablet and preparation method thereof
A technology of cefpodoxime axetil and dispersible tablets, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., and can solve problems such as unfavorable industrial production, complex preparation process, and many equipments , to achieve the effect of good disintegration time limit, simple process and good content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] prescription:
[0033]
[0034] Preparation:
[0035] 1) Take the above cefpodoxime axetil and pass through a 100-mesh sieve, and pass the auxiliary materials through a 80-mesh sieve respectively, and set aside.
[0036] 2) Put 350g of cefpodoxime axetil, 450g of microcrystalline cellulose, 75g of croscarmellose sodium, 2005g of polyethylene glycol, and 50g of magnesium stearate in a wet granulator and mix for 30 minutes, then place in a dry Granulate in a French granulator, then add 75g of croscarmellose sodium, 50g of magnesium stearate, and 5g of stevia, mix well, and press into tablets with a tablet machine.
Embodiment 2
[0038] prescription:
[0039]
[0040]
[0041] Preparation:
[0042] 1) Take the above cefpodoxime axetil and pass through a 100-mesh sieve, and pass the auxiliary materials through a 80-mesh sieve respectively, and set aside.
[0043] 2) Put 700g of cefpodoxime axetil, 900g of microcrystalline cellulose, 150g of sodium carboxymethyl starch, 20010g of polyethylene glycol, and 100g of magnesium stearate in a wet granulator and mix for 30 minutes, then put it in a dry granulator Medium granulation, then add 150g of sodium carboxymethyl starch, 100g of magnesium stearate, 5g of stevioside, mix evenly, and press into tablets with a tablet machine.
Embodiment 3
[0045] prescription:
[0046]
[0047] Preparation:
[0048] 1) Take the above cefpodoxime axetil and pass through a 100-mesh sieve, and pass the auxiliary materials through a 80-mesh sieve respectively, and set aside.
[0049] 2) Put 350g of cefpodoxime axetil, 450g of pregelatinized starch, 75g of croscarmellose sodium, 2005g of polyethylene glycol, and 50g of talcum powder in a wet granulator and mix for 30 minutes, then dry granulate Granulate in the machine, add 75g of croscarmellose sodium, 50g of talcum powder, and 5g of aspartame, mix evenly, granulate, and press into tablets with a tablet machine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com