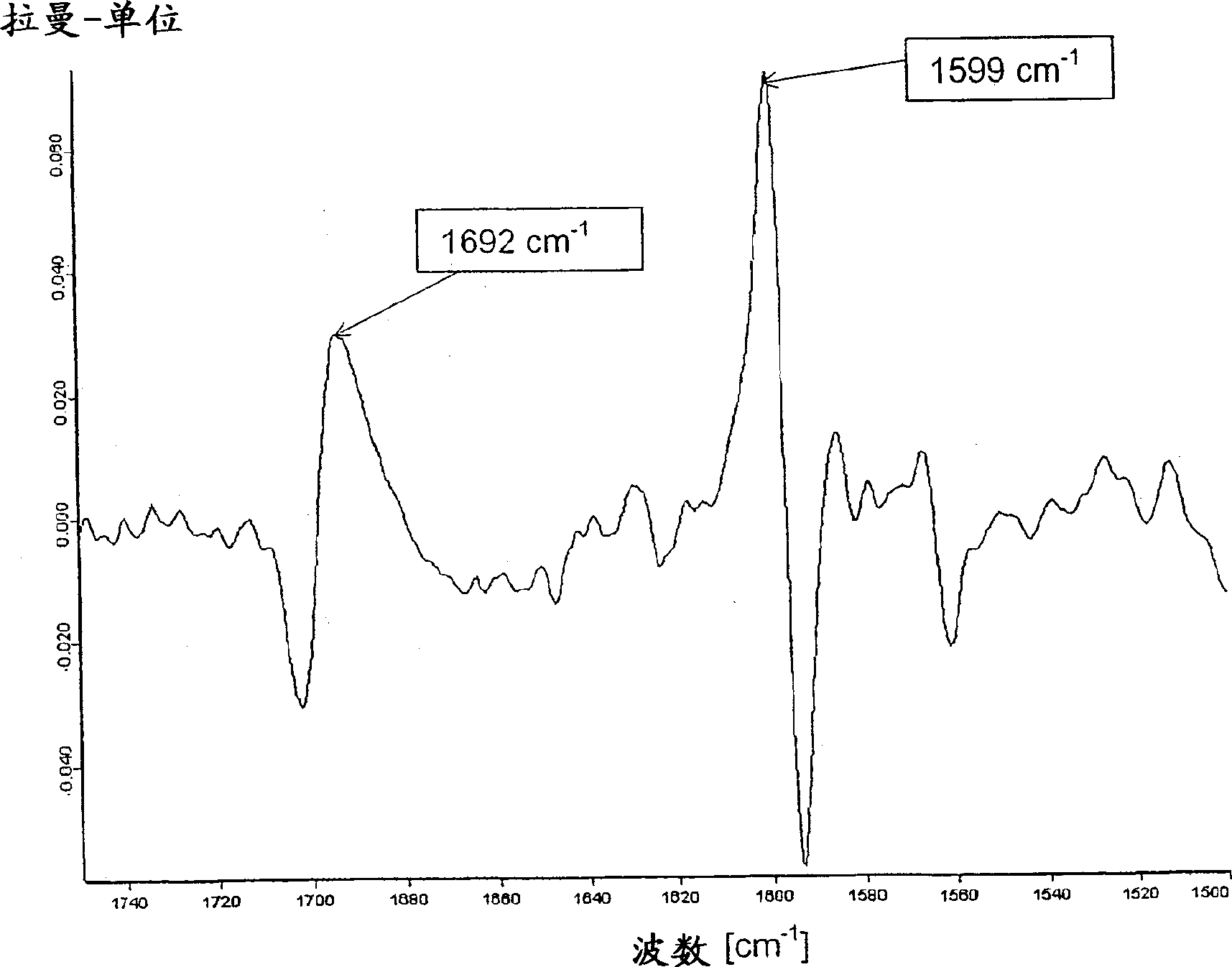
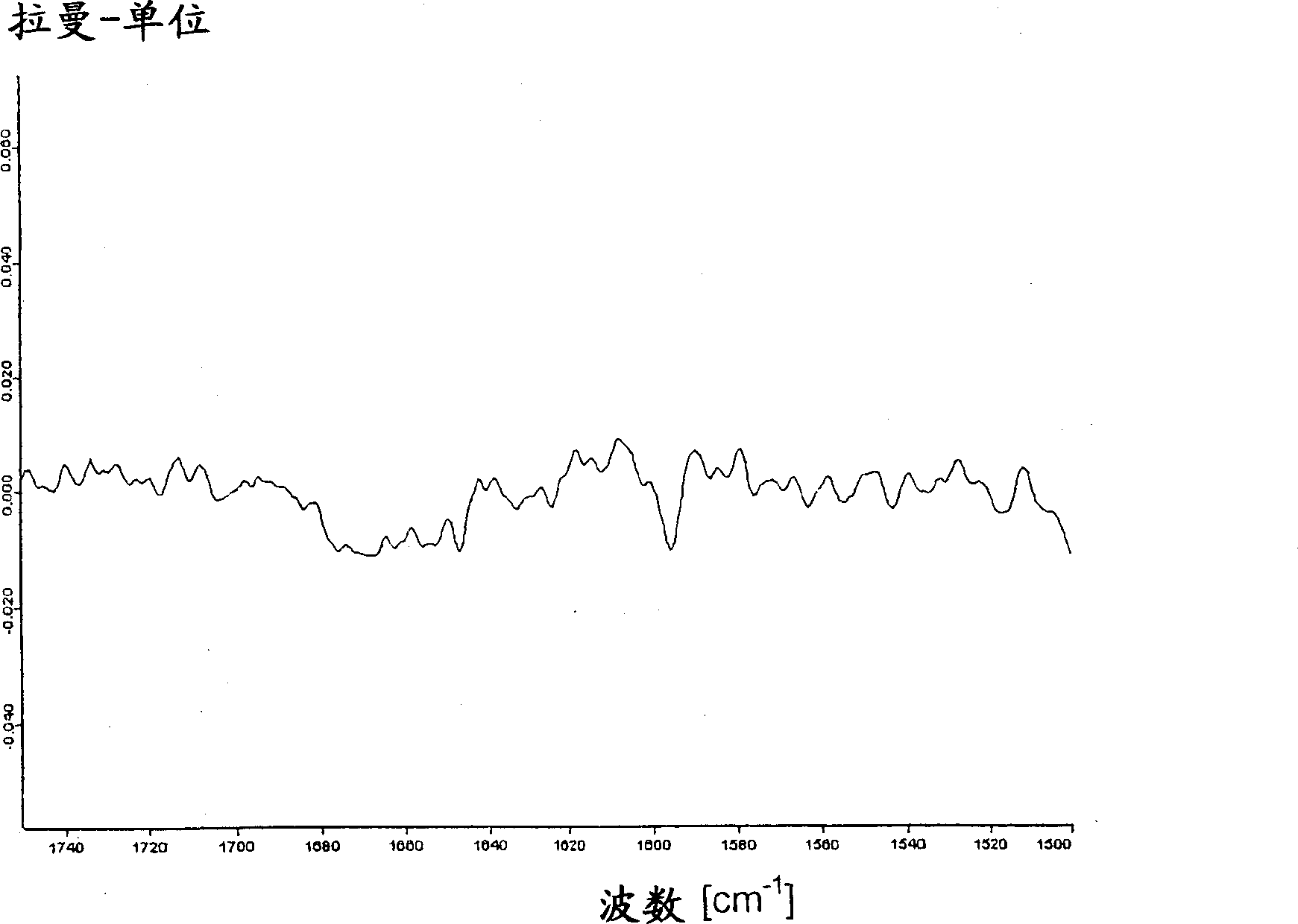
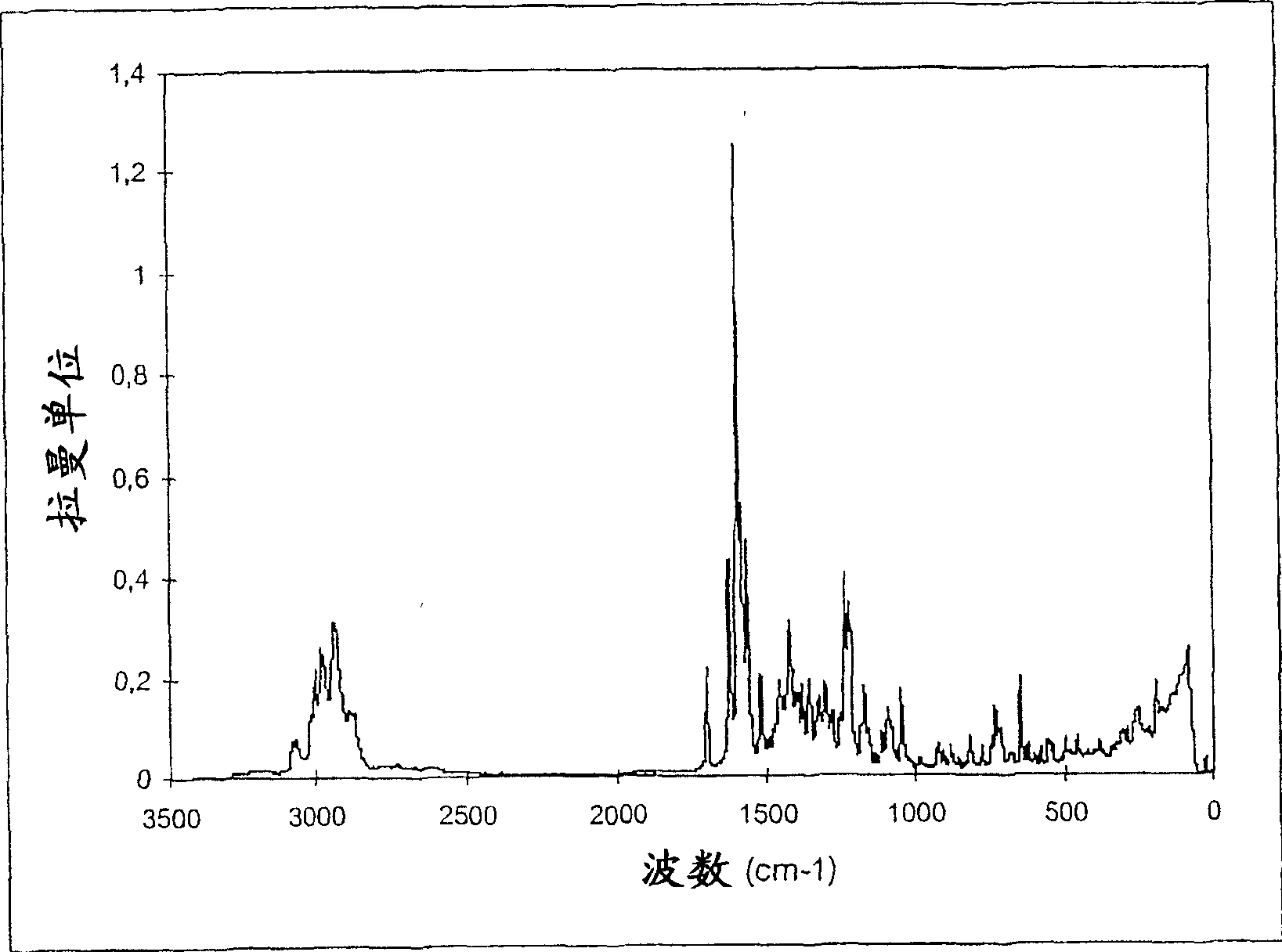
Legemiddel inneholdende vardenafilhydrokloridtrihydrat
A technology of vardenafil hydrochloride and trihydrate, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, sugar-coated pills, etc., can solve problems such as inability to separate vardenafil HCl, and achieve rapid disintegration and clear definition , the effect of fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0058] The tablet has a 150m 3 / h Rehydration for 4 hours in a fluidized bed granulator with inlet air at a temperature of 30° C. and a water content of 19 g / kg (corresponds to a relative air humidity of 70%). Therefore, the modified form of vardenafil HCl in the tablet of the present invention is equivalent to the trihydrate. Tablets now disintegrate in just 1 / 2 minute.
Embodiment 3
[0059] Example 3: The stability of the prepared tablet of the present invention
[0060] 28.4 kg of micronized vardenafil HCl trihydrate were mixed with 69.6 kg of microcrystalline cellulose and 5.16 kg of sieved crospovidone in a mechanical mixer. This mixture was mixed with 182 kg microcrystalline cellulose and 9.84 kg crospovidone in a tank mixer and granulated by drum dry granulation. After mixing 1.50 kg of highly disperse silicon dioxide and 3.00 kg of magnesium stearate, tablets with a mass of 125 mg and a diameter of 7 mm were prepared with a rotary tablet press. The uncoated tablet was coated with 5.74 kg hypromellose, 1.91 kg polyethylene glycol 400, 1.57 kg titanium dioxide, 319 g yellow iron oxide, 25.5 g red iron oxide in a commercially available coating system And the suspension of 118kg purified water carries out coating. The modified form of vardenafil HCl in this coated tablet does not correspond to the trihydrate form, therefore, it represents an undefine...
Embodiment 4
[0062] Example 4: Release of Active Ingredient from Tablets Prepared According to the Invention
[0063] 336g vardenafil HCl was mixed with 2216g microcrystalline cellulose and 134g crospovidone and it was dry granulated. The granules were transferred, then mixed with 283 g microcrystalline cellulose, 16 g crospovidone and 15 g magnesium stearate and compressed to a diameter of 5 mm and a mass of 48 mg (equivalent to 5 mg vardenafil base per tablet ) tablets. The tablet was provided with a white coating of 57.4 g hypromellose, 19.1 g macrogol 4000 and 19.1 g titanium dioxide and stored in an oven at 25° C. and a relative air humidity of 80% for 4 sky. The modification of vardenafil HCl in the tablet corresponds to the trihydrate form.
[0064] As shown by the release data in Table 1, the tablets of the invention prepared in this way released the active very rapidly, although all of the vardenafil HCl in the final tablet reverted to the trihydrate form. Element.
[0065]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com