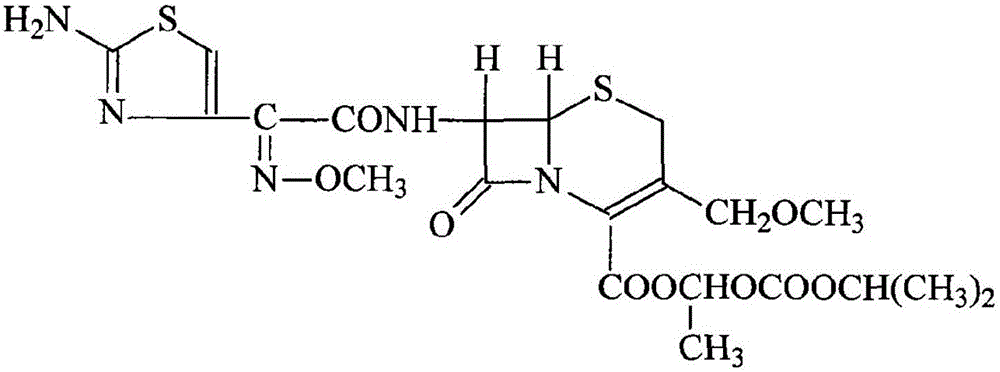
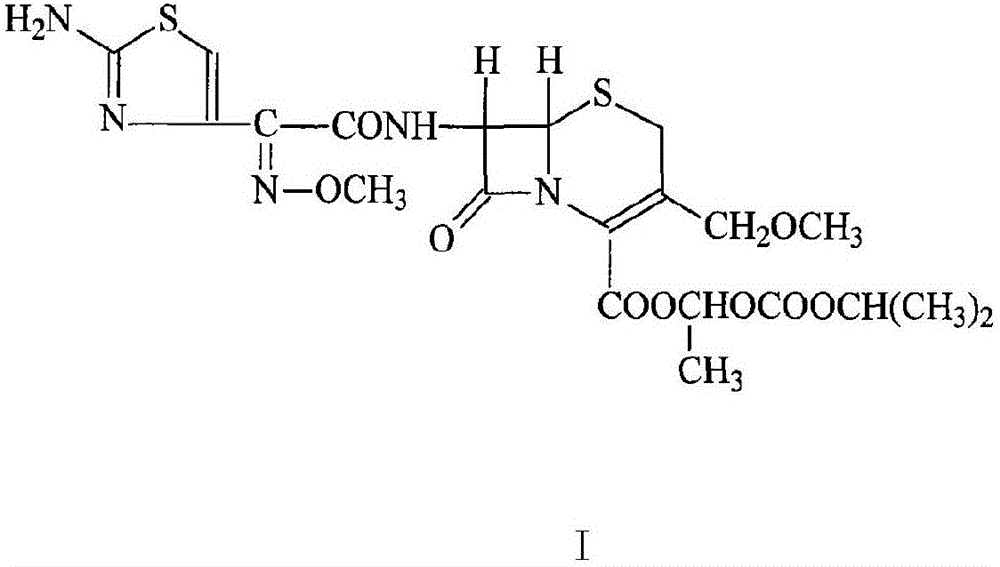
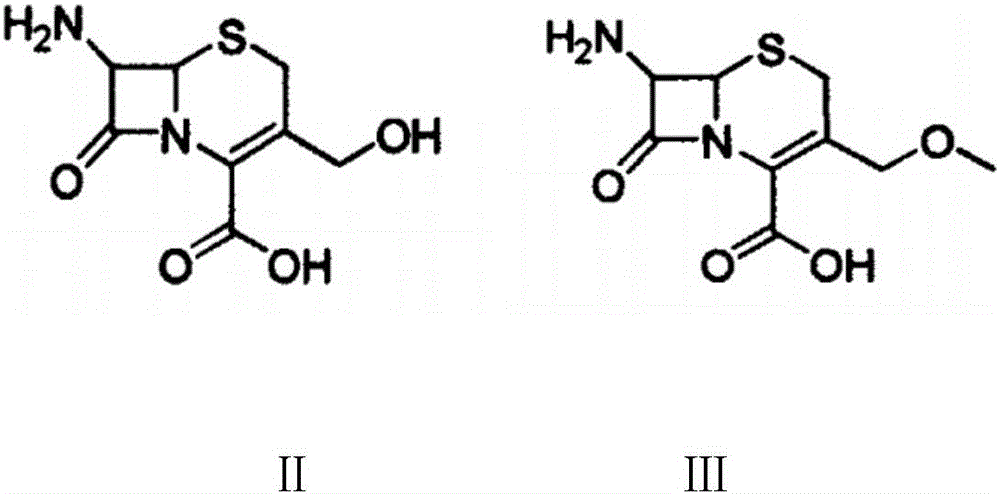
Preparation method of cefpodoxime proxetil
A technology of cefpodoxime axetil and active ester is applied in the field of preparation of cefpodoxime axetil, can solve the problems of high content of Δ2 isomers, cannot reach preparations, low total yield, etc., and achieves simple synthetic route and guaranteed The effect of improving safety and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Take 220mL of trimethyl orthoformate in a round-bottomed beaker, cool to -30°C, add dropwise 500mL of a mixture of boron trifluoride ether and acetonitrile, mix at a volume ratio of 1:1, and add dropwise while stirring. After dropping within 45 minutes, continue to stir for 50 minutes, add 240g of D-7-ACA raw material, stir for 15 minutes, heat it up to 25°C in a water bath, monitor the reaction with TLC, react for 5 to 6 hours, and the reaction is basically completed;
[0036] (2) Evaporate acetonitrile, wash twice with anhydrous ether ultrasonically, pour off the ether, add 2500mL dichloromethane, stir at 25°C, then add 100mL triethylamine at 0°C, adjust the pH to 8-9, Then add 378g of AE-active ester (MAEM) in three times respectively, and each time the active ester is added, adjust the pH to 8-9 with triethylamine again, and continue to react at 0° C. for 6-7 hours. After the reaction, add 3L of water, separate the layers, collect the water layer, adjust the pH ...
Embodiment 2
[0039] (1) Take 110mL trimethyl orthoformate in a round bottom beaker, cool to -20°C, add dropwise 250mL of a mixture of boron trifluoride ether and acetonitrile, mix at a volume ratio of 1:1, and add dropwise while stirring, After dropping within 25 minutes, continue to stir for 30 minutes, add 120g of D-7-ACA raw material, stir for 15 minutes, heat it up to 25°C in a water bath, monitor the reaction with TLC, react for 3 to 4 hours, and the reaction is basically completed;
[0040] (2) Evaporate acetonitrile, wash twice with anhydrous ether ultrasonically, pour off the ether, add 1500mL dichloromethane, stir at 25°C, then add 50mL triethylamine at 5°C, adjust the pH to 8-9 , and then add 189g of AE-active ester (MAEM) three times respectively, and each time the active ester is added to adjust the pH to 8-9 with triethylamine again, and continue to react at 5°C for 4-5 hours. After the reaction, add 1.5 L of water, separate layers, collect the water layer, adjust the pH value...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com