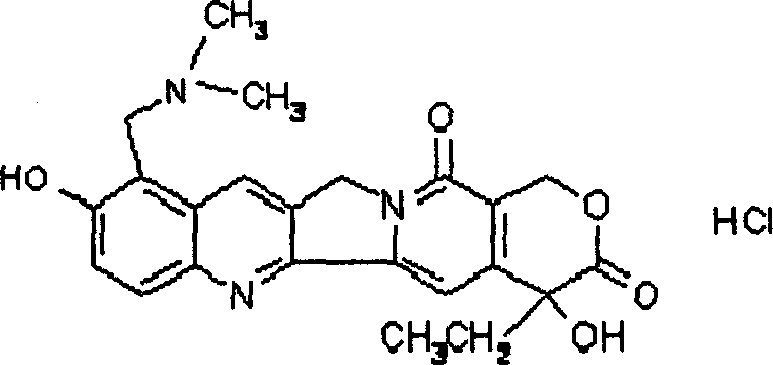
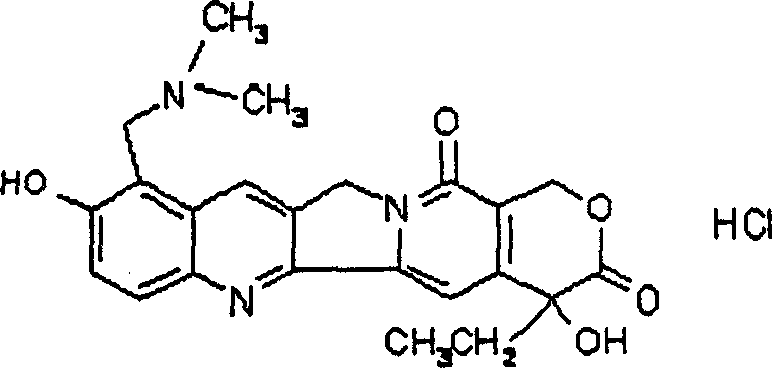
Topotecan liposome and preparation method therefor
A technology of topotecan and topotecan hydrochloride, which is applied in liposome delivery, pharmaceutical formula, liquid delivery, etc., can solve the problems affecting the treatment effect of topotecan, patient pain, no improvement, etc., and improve the drug therapy index , relieve pain, reduce the effect of thrombocytopenia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take topotecan hydrochloride and prepare 1 mg / ml topotecan hydrochloride solution with phosphate buffer. Another 2 g of hydrogenated soybean lecithin was dissolved in ethanol, slowly dripped in 50 ml of topotecan hydrochloride solution kept at 55-65° C. under stirring (the weight ratio of topotecan and soybean lecithin was 1: 40), Ethanol was evaporated, and stirring was continued for 2h to obtain topotecan liposomes.
Embodiment 2
[0026] Take topotecan hydrochloride and prepare 1 mg / ml topotecan hydrochloride solution with phosphate buffer. Put 1g of soybean lecithin and 0.3g of cholesterol into a round-bottomed flask, add 20ml of absolute ethanol, heat in a water bath at 65-70°C, stir, fully dissolve, then rotate on a rotary evaporator to form a film, remove the ethanol under reduced pressure to obtain a phospholipid film . Get 50ml of topotecan hydrochloride solution (the weight ratio of topotecan and soybean lecithin is 1: 20), pour it into the above-mentioned flask, heat and stir in a water bath at 65~70° C. Stir for 60 minutes to obtain topotecan liposomes.
Embodiment 3
[0028] Get topotecan hydrochloride, and make 2 mg / ml topotecan hydrochloride solution with phosphate buffer. Put 1g of soybean lecithin and 0.3g of cholesterol into a round-bottomed flask, add 20ml of absolute ethanol, heat in a water bath at 65-70°C, stir, fully dissolve, then rotate on a rotary evaporator to form a film, remove the ethanol under reduced pressure to obtain a phospholipid film . Get 50ml of topotecan hydrochloride solution (the weight ratio of topotecan and soybean lecithin is 1: 10), pour it into the above-mentioned flask, heat and stir in a water bath at 65~70° C. Stir for 60 minutes to obtain topotecan liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com