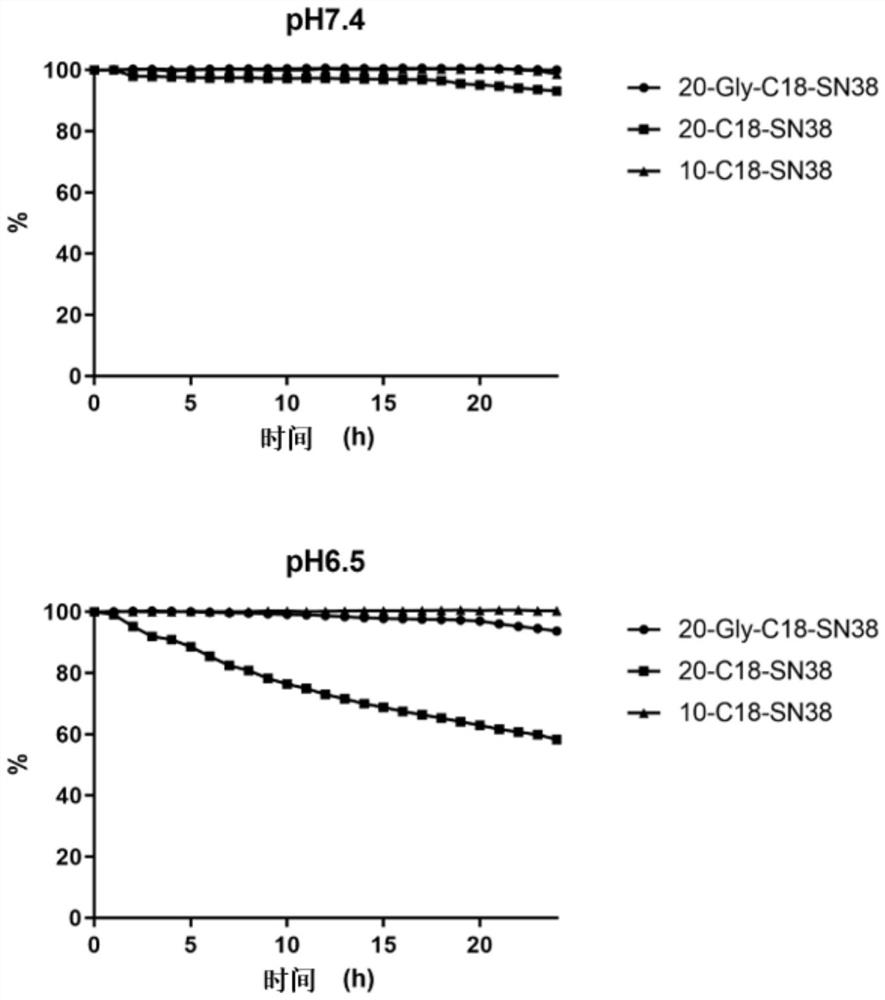
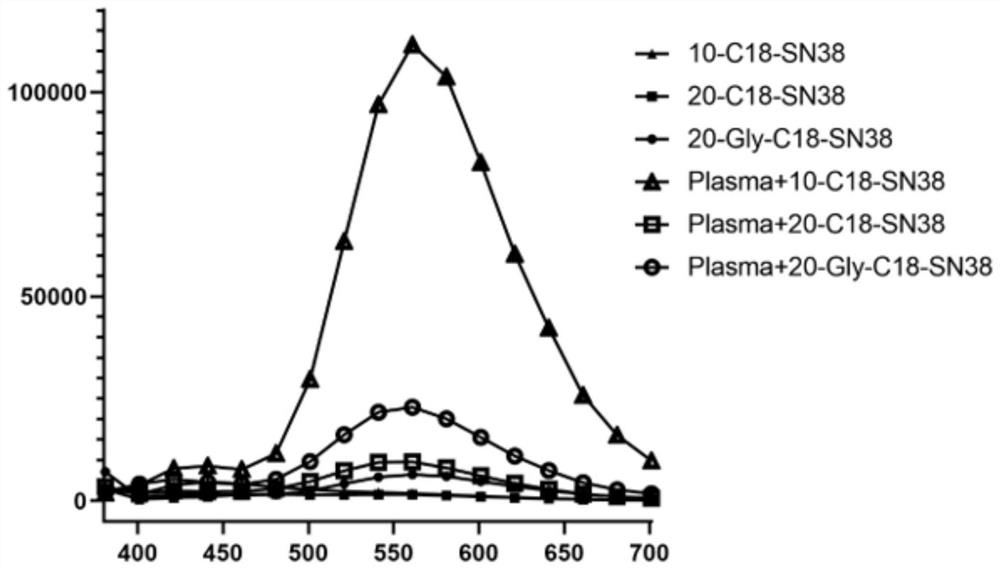
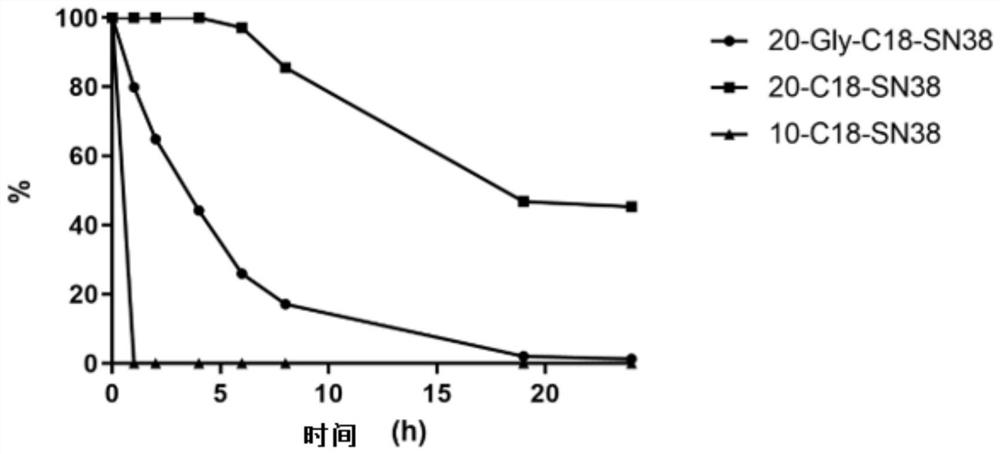
7-ethyl-10-hydroxycamptothecin derivative prodrug non-covalently bound with human serum albumin, and preparation and application of 7-ethyl-10-hydroxycamptothecin derivative prodrug
A technology of human serum albumin and hydroxycamptothecin, applied in the field of biomedicine, can solve the problems of no targeting, low solubility of SN38, and limited clinical application, etc., and achieve improved physical and chemical properties, small side effects, and effective anti-tumor effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of Compound 10-C18-SN38
[0051]
[0052] Preparation of compound 1:
[0053] Under nitrogen protection conditions, 0.5g (1.27mmol) SN38, 0.59g (1.59mmol) mono-tert-butyl octadecanedioate, 0.66g (3.44mmol) EDCI and 0.04g (0.29mmol) DMAP were dissolved in 15mL of anhydrous di In methyl chloride, react for 4 hours. Diluted by adding 15 mL of dichloromethane, saturated ammonium chloride (NH 4 Cl) solution (15 mL), water (15 mL), saturated brine (15 mL), washed with anhydrous sodium sulfate, and spin-dried. Purify by silica gel column chromatography, the mobile phase is dichloroalane:methanol=200:1-90:1. The collected pure product was compound 1, which was spin-dried to obtain 0.48 g of light yellow solid with a yield of 51.1%.
[0054] 1 H NMR (400MHz, CDCl 3 )δ8.23(d, J=9.1Hz, 1H), 7.79(s, 1H), 7.66(d, J=9.1Hz, 1H), 7.53(s, 1H), 5.74(s, 2H), 5.24( s,2H),3.14(q,J=7.3Hz,2H),2.66(m,2H),2.20(m,2H),1.98–1.87(m,2H),1.85–1.80(m,2H),1.61 –1.53(m,2H),1.44(s,9H...
Embodiment 2
[0060] Synthesis of Compound 20-C18-SN38
[0061]
[0062] Preparation of compound 2-1:
[0063] Under nitrogen protection conditions, 1.51g (3.84mmol) SN38, 1.09g (4.99mmol) Boc 2 O and 9 mL (112.87 mmol) of anhydrous pyridine were dissolved in 22 mL of anhydrous dichloromethane, and stirred overnight at room temperature. With 0.5M dilute hydrochloric acid (HCl) solution (10mL), saturated sodium bicarbonate (NaHCO 3 ) solution (10 mL), saturated brine (10 mL), and dried over anhydrous sodium sulfate. Evaporated to dryness under reduced pressure to obtain 1.85 g of compound 2-1 as a yellow solid, with a yield of 98%.
[0064] 1 H NMR (400MHz, CDCl 3 )δ8.24(d, J=9.2Hz, 1H), 7.89(s, 1H), 7.66(d, J=6.1Hz, 2H), 5.75(d, J=16.3Hz, 1H), 5.34–5.25( m,3H),3.87(s,1H),3.16(q,J=7.5Hz,2H),1.96–1.85(m,2H),1.62(s,9H),1.40(t,J=7.5Hz,3H ), 1.04(t,J=7.3Hz,3H).
[0065] Preparation of Compound 2-2:
[0066] Under nitrogen protection, 0.5g (1.02mmol) compound 2-1, 0.56g (1.52mmol) mon...
Embodiment 3
[0073] Synthesis of compound 20-Gly-C18-SN38:
[0074]
[0075] Preparation of compound 2-1:
[0076] Under nitrogen protection conditions, 1.51g (3.84mmol) SN38, 1.09g (4.99mmol) Boc 2 O and 9 mL (112.87 mmol) of anhydrous pyridine were dissolved in 22 mL of anhydrous dichloromethane, and stirred overnight at room temperature. With 0.5M HCl solution (10mL), saturated NaHCO 3 solution (10 mL), washed with saturated brine (10 mL), and dried over anhydrous sodium sulfate. Evaporated to dryness under reduced pressure to obtain 1.85 g of compound 2-1 as a yellow solid, with a yield of 98%.
[0077] 1 H NMR (400MHz, CDCl 3 )δ8.24(d, J=9.2Hz, 1H), 7.89(s, 1H), 7.66(d, J=6.1Hz, 2H), 5.75(d, J=16.3Hz, 1H), 5.34–5.25( m,3H),3.87(s,1H),3.16(q,J=7.5Hz,2H),1.96–1.85(m,2H),1.62(s,9H),1.40(t,J=7.5Hz,3H ), 1.04(t,J=7.3Hz,3H).
[0078] Preparation of compound 3-1:
[0079] Under nitrogen protection, 0.5g (1.02mmol) of compound 2-1, 0.36g (2.03mmol) of N-Boc-glycine, 0.23g (1.22mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com