Method for jointly preparing CAR-NK (chimeric antigen receptor-natural killer) cells and CAR-NKT (natural killer T) cells
A technology of NKT cells and NK cells, which is applied in the field of joint preparation of CAR-NK cells and CAR-NKT cells, can solve the problems of immune rejection, low transduction efficiency of allogeneic CAR-T, and low ratio, so as to save purification costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
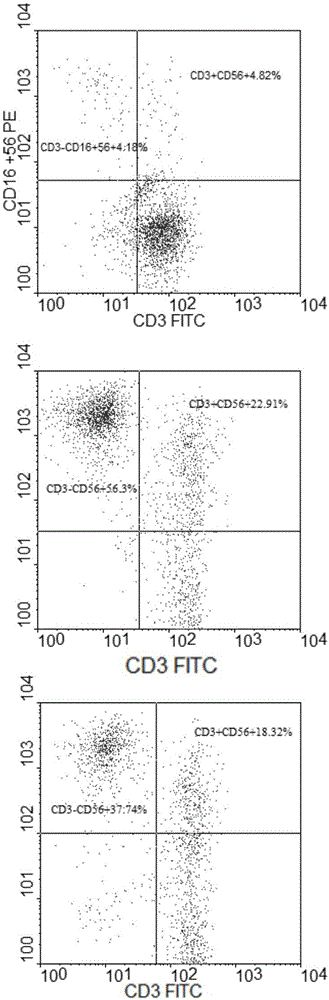
[0046] PBMCs were isolated and counted from 80ml of peripheral blood of healthy donors using human lymphocyte separation medium Ficoll paque plus (GE) in the ultra-clean workbench of the GMP laboratory, and a small amount of samples were taken for immunophenotypic analysis before PBMC culture, see figure 1 Add the PBMCs to the serum-free cell culture medium AIM-V (GIBCO, U.S.) containing 10% plasma (plasma and peripheral blood come from the same healthy donor) after being suspended, and use the above-mentioned serum-free cells containing plasma to obtain the cell concentration. Medium adjusted to 2×10 6 cells / ml, and then all transferred to culture flasks coated with 20 μg / ml anti-CD16 monoclonal antibody, preactivated NK cells and NKT cells in an incubator at 37°C and 5% carbon dioxide, and added after 24 hours of preactivation Add IL-2 to make the concentration in the serum-free cell culture medium 1000U / ml, and add IL-15 to make the concentration in the serum-free cell cult...
Embodiment 2
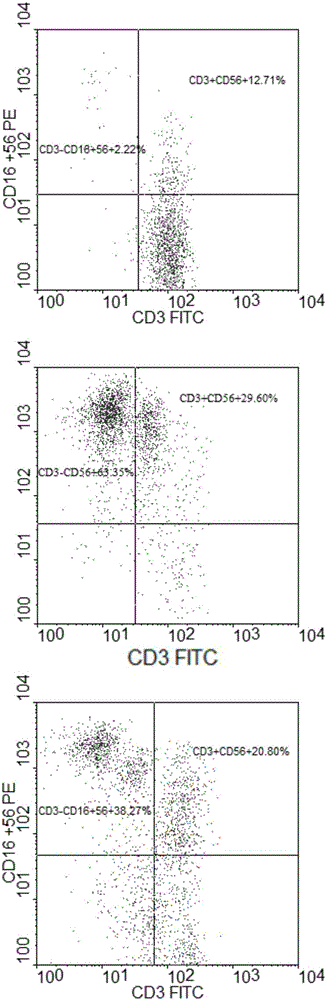
[0050] In the ultra-clean workbench of the GMP laboratory, human lymphocyte separation medium Ficoll paque premium (GE) was used to separate and count PBMCs from 80ml of peripheral blood of healthy donors. A small amount of samples were taken for immunophenotyping analysis before PBMC culture, see figure 2 Add the PBMCs to the serum-free cell culture medium AIM-V (GIBCO, U.S.) containing 10% plasma (plasma and peripheral blood come from the same healthy donor) after being suspended, and use the above-mentioned serum-free cells containing plasma to obtain the cell concentration. Medium adjusted to 2 × 10 6 cells / ml, and then all transferred to a culture flask co-coated with 10ng / ml anti-CD3 monoclonal antibody and 10μg / ml anti-CD52 monoclonal antibody, and pre-activated in an incubator at 37°C and 5% carbon dioxide NK cells and NKT cells, after preactivation for 24h, add IL-2 to make the concentration in the serum-free cell culture medium 1000U / ml and add IL-15 to make the con...
Embodiment 3
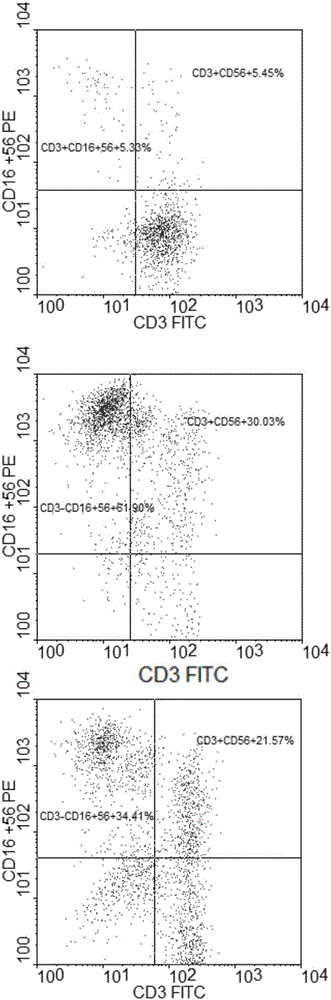
[0054] In the ultra-clean workbench of the GMP laboratory, human lymphocyte separation medium Ficoll paque premium (GE) was used to separate and count PBMCs from 80ml of peripheral blood of healthy donors. A small amount of samples were taken for immunophenotyping analysis before PBMC culture, see image 3 After PBMC is suspended, add in the serum-free cell culture medium AIM-V (U.S. GIBCO company) that contains 10% plasma (plasma and peripheral blood come from the same patient), the cell concentration is replaced with the above-mentioned serum-free cell culture medium that contains plasma Adjust to 2×10 6 cells / ml, and then all transferred to the culture bottle, and then add the trophoblast cell K562-4-1BB-Mil-21 (purchased from Youkang Bio) to the culture bottle to make the final concentration in the serum-free cell culture medium 1× 10 5 cells / ml, preactivate NK cells and NKT cells in an incubator at 37°C and 5% carbon dioxide, add IL-2 after 24 hours of preactivation to m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com