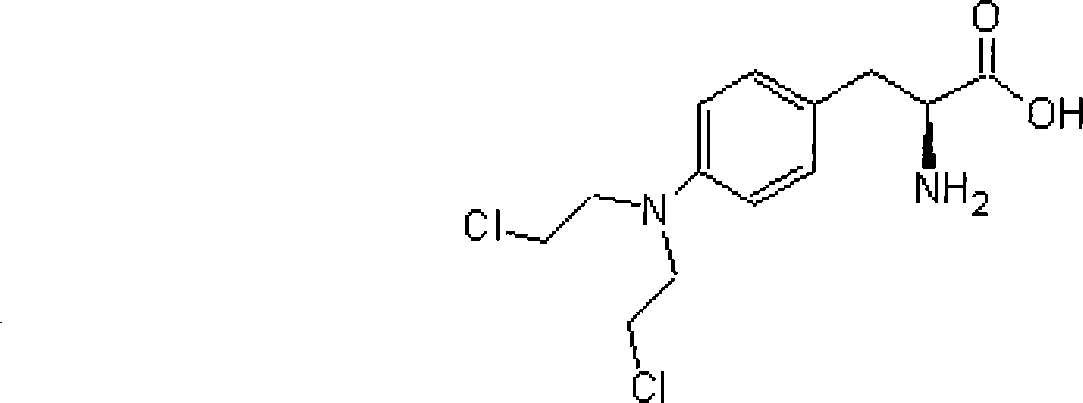
Technique for synthesizing antineoplastic melphalan
An anti-tumor drug and synthesis process technology, applied in the field of anti-tumor drug melphalan synthesis process, can solve the problems of unfavorable industrial production, increased reaction cost, cumbersome operation process, etc. The effect of processing operations and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
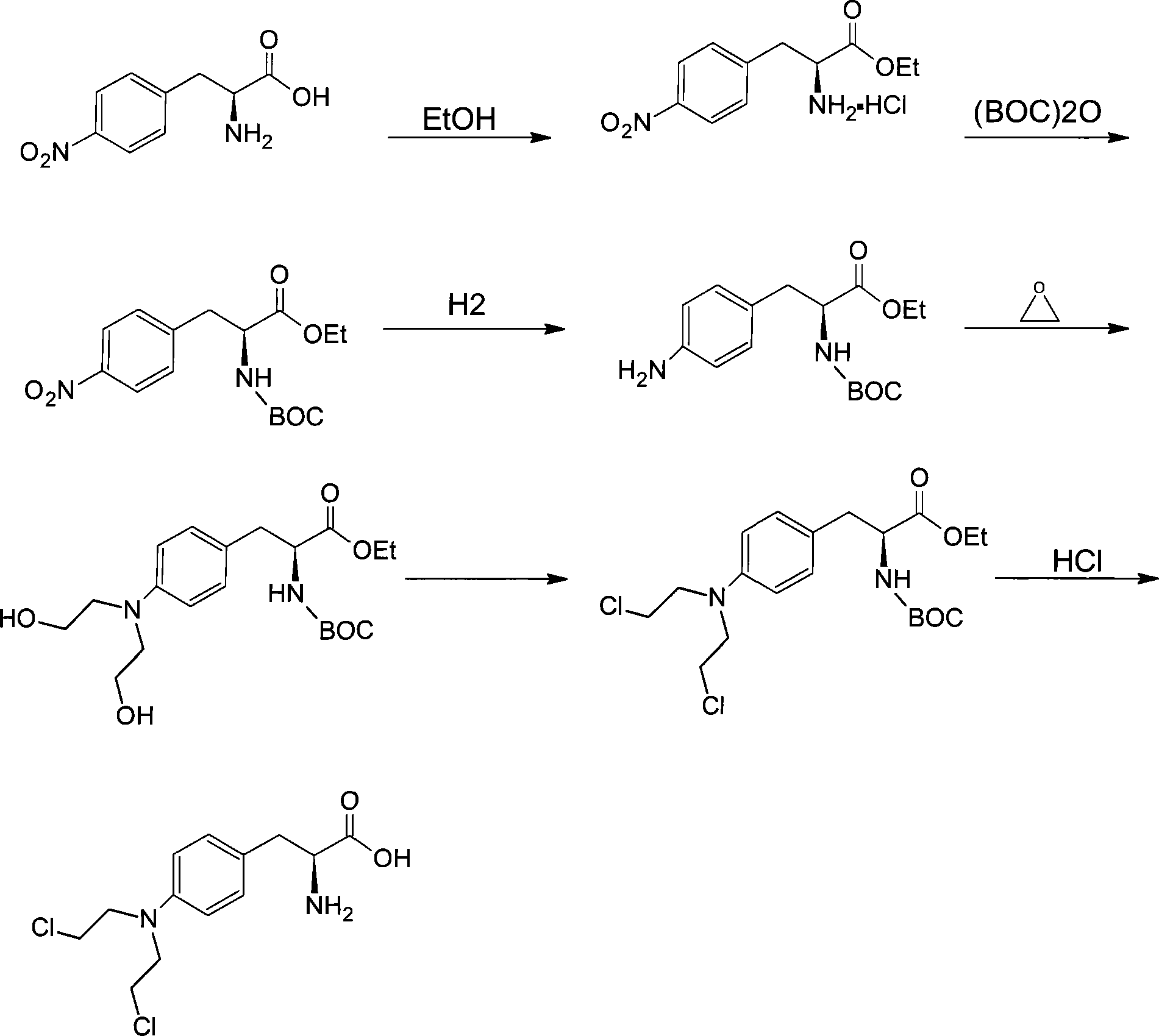
Examples
Embodiment 1
[0027] Step (1) esterification reaction:
[0028] Take 100 grams of L-4-nitrophenylalanine and 1000 milliliters of absolute ethanol and mix them in a four-neck flask, add 51 milliliters of thionyl chloride dropwise while stirring, and heat to reflux at 78°C after the addition is complete After 2.5 hours, stop heating, slowly cool to 10°C, filter with suction, and dry the filter cake under an infrared lamp to obtain 107 g of L-4-nitrophenylalanine ethyl ester, the yield of this step is 82.3%.
[0029] Step (2) amino protection reaction:
[0030] Take 100 grams of L-4-nitrophenylalanine ethyl ester and 1800 milliliters of dichloromethane and mix them in a three-necked flask, add 60 milliliters of triethylamine, add 80 grams of di-tert-butyl dicarbonate under normal temperature stirring, and stir slowly After 8 hours, the reaction solution was washed twice with 770 milliliters of 1 mol / liter hydrochloric acid solution each time, and then washed three times with 800 milliliters o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com