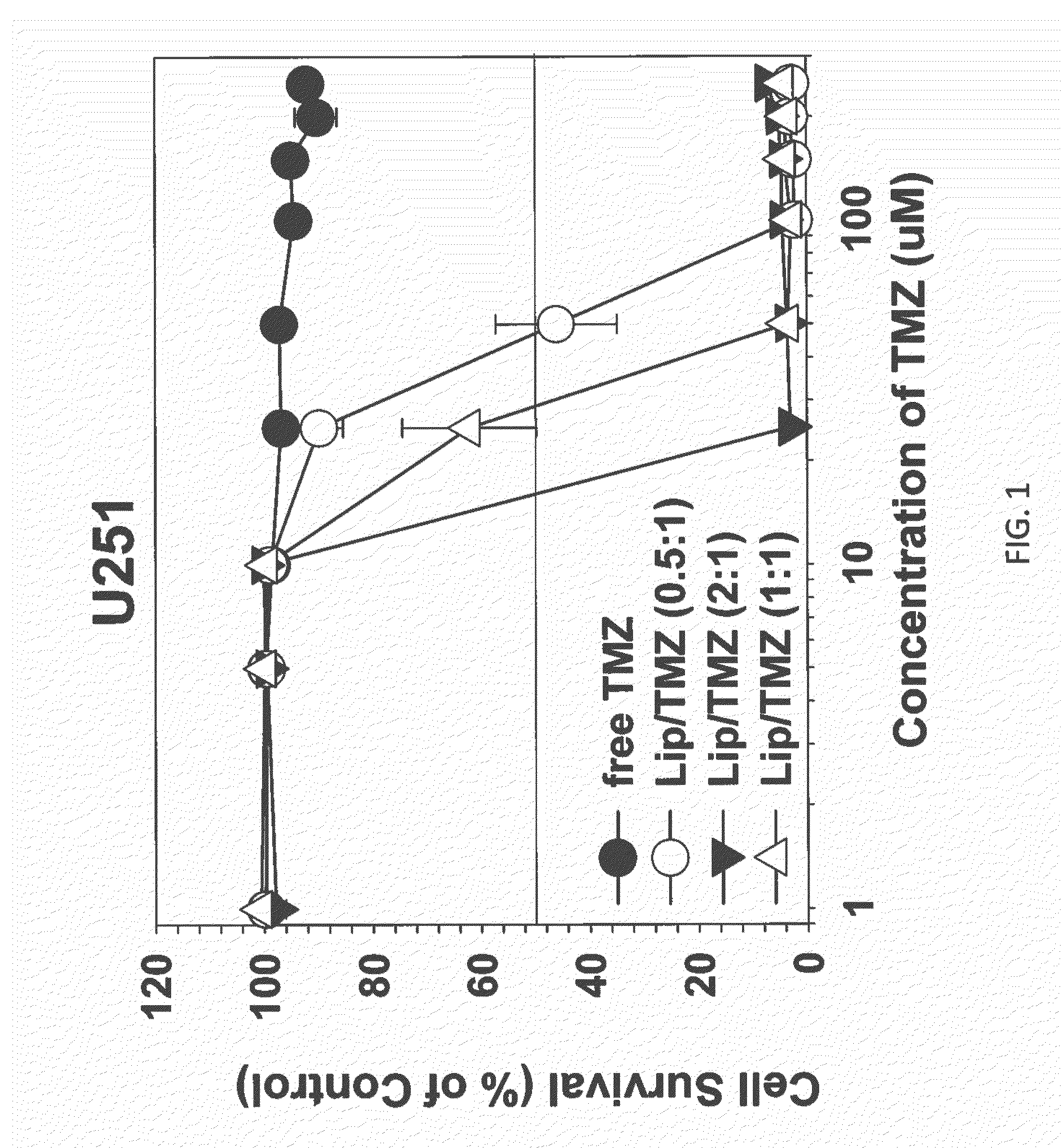
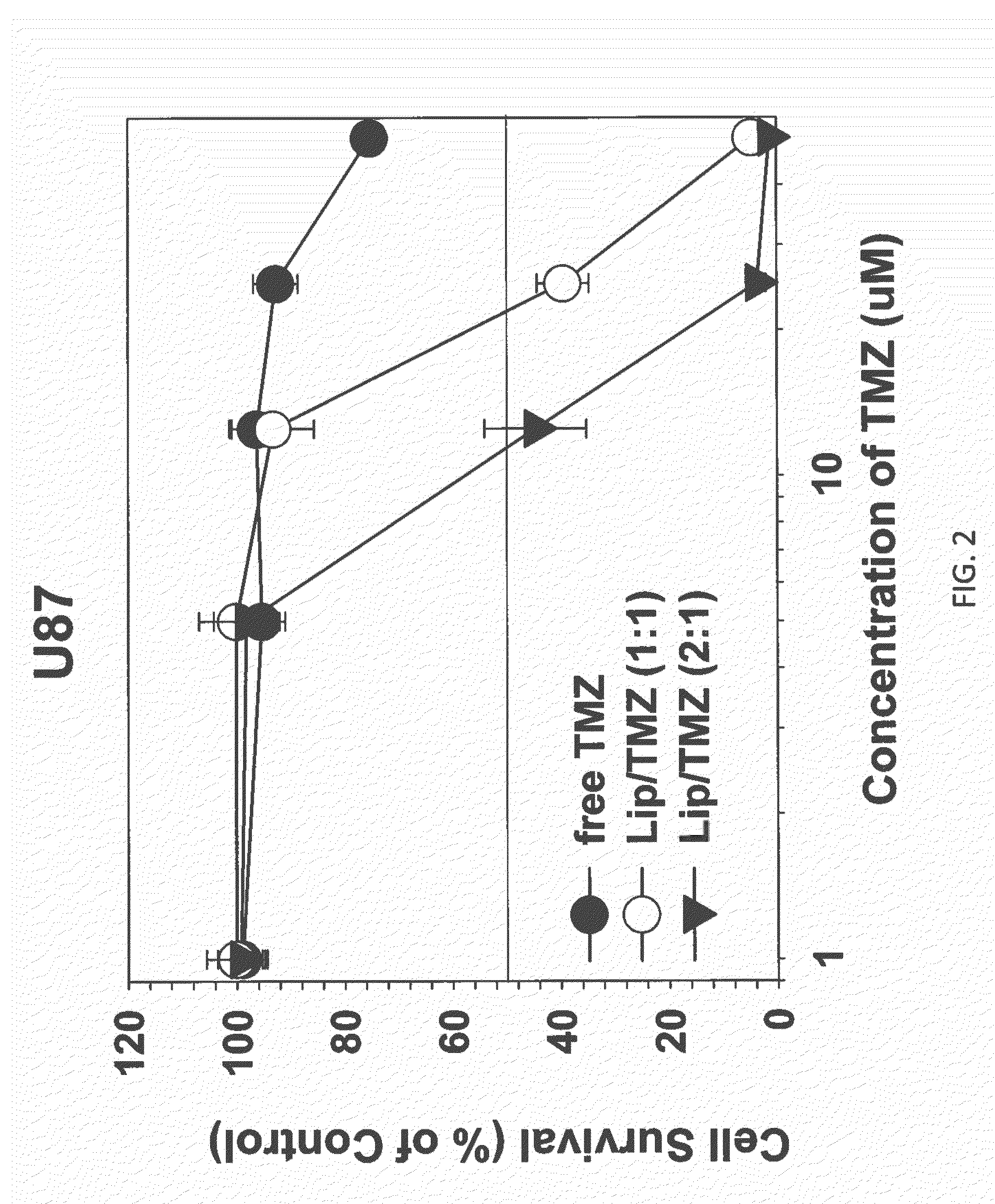
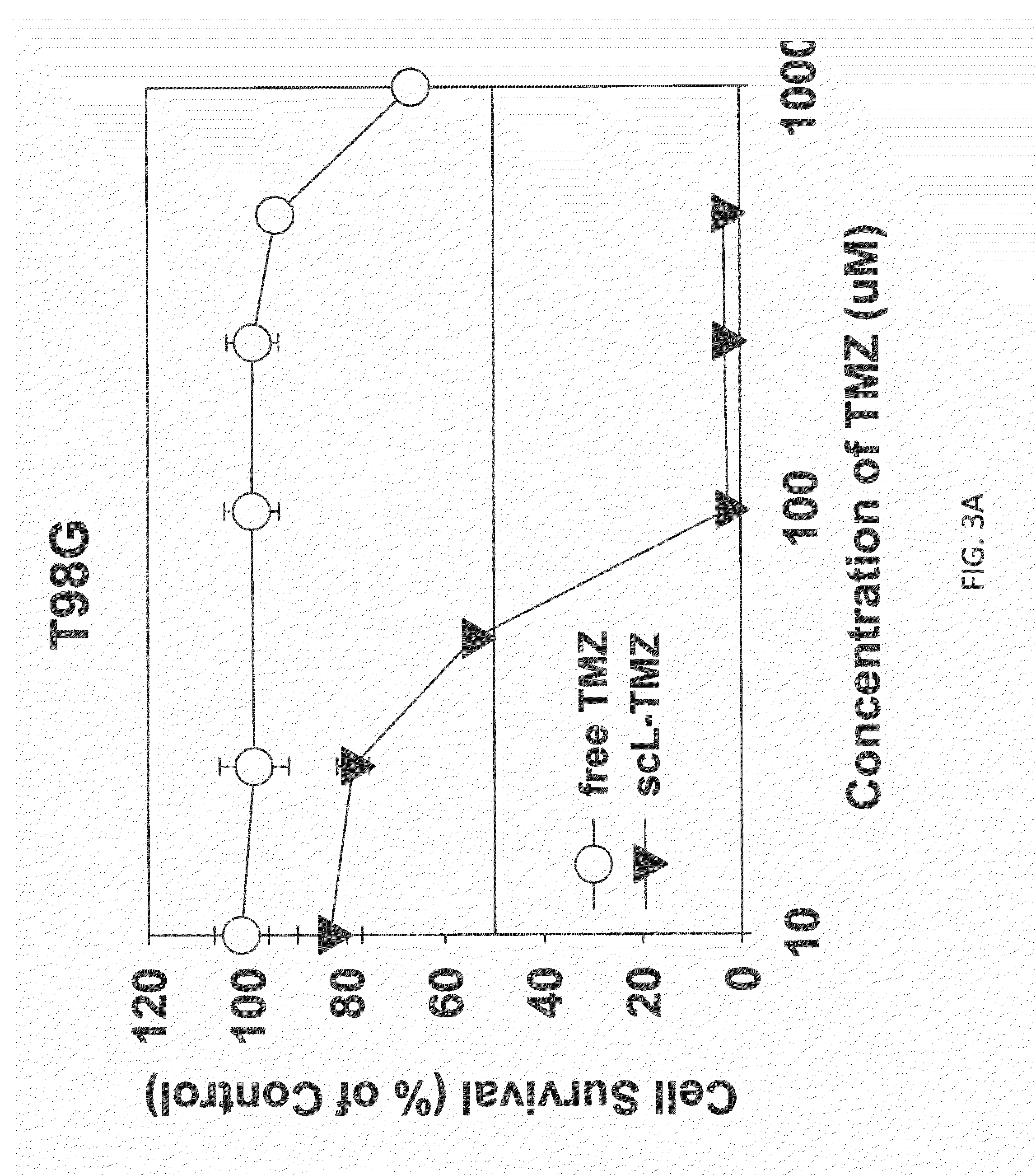
Targeted liposomes
a liposome and target technology, applied in the field of drug delivery, can solve the problems of lack of improvement in limited therapeutic dosage, and difficult treatment of primary brain tumors, and achieve the effects of improving the prognosis of brain cancer patients, improving the prognosis, and improving the treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cationic Liposomes Comprising Temozolomide
[0149]Materials:
[0150]DOTAP (1,2-dioleoyl-3-trimethylammonium propane, chloride salt)[0151]Obtained from Avanti Polar Lipids, Inc. Cat. #890890E, MW 698.55[0152]Concentration: 25 mg / mL ethanol solution[0153]Dilute lipid to 20 mg / ml with absolute ethanol before use
[0154]DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine)[0155]Obtained from Avanti Polar Lipids, Inc. Cat. #850725E, MW 744.04[0156]Concentration: 25 mg / mL ethanol solution.[0157]Dilute lipid to 20 mg / ml with absolute ethanol before use
[0158]Temozolomide (TMZ, M.W. 194.15), powder[0159]Obtained from Sigma, Cat. #T2577-100 mg[0160]Dissolve TMZ in pure DMSO to desired concentration. For example, 19.415 mg / ml=100 mM of TMZ; 28 mg / ml=144.218 mM of TMZ
[0161]Ultra-pure, endotoxin free LAL Reagent Water (e.g. BioWhittaker, Cat. #W50-500, endotoxin <0.005 EU / ml)
[0162]Injector: Hamilton Gastight Syringe, 1 ml (Hamilton #81230) with a 22 gauge needle, part #81365)
Procedure:
[0...
example 2
Preparation of scL-TMZ without Chemical Conjugation (by Simple Mixing)
[0193]Using the TMZ-comprising cationic liposomes prepared according to the procedure described in Example 1, the ligand targeted TMZ cationic liposome complex as described herein is prepared by simple mixing of the components and without chemical conjugation. The preparation of the complexes was in accordance with the following general procedure:
[0194]To the liposome-water (or buffer) the appropriate amount of targeting moiety is added to give the desired ratio and mixed by gentle inversion 5-10 seconds. The targeting moiety can be a ligand including but not limited to transferrin or folate, or other proteins. It can also be an antibody or an antibody fragment that targets a cell surface receptor including, but not limited to the transferrin or HER-2 receptor (e.g., TfRscFv). This mixture is kept at room temperature for 10-15 minutes (again inverted gently for 5-10 seconds after approximately 5 minutes). To yield...
example 3
Determination of the Percent Encapsulation of TMZ in the scL-TMZ Complex
[0200]To determine the percent of the TMZ encapsulated in the scL-TMZ complex, we prepared scL-TMZ as described in Example 1 with 2 mM stock Lip:TMZ. Various amounts of complex were prepared ranging from 126 to 560 ul scL-TMZ. The scL-TMZ complex was subsequently diluted to a Lip:TMZ concentration of 0.5 mM. The initial concentration of TMZ was checked by measuring the absorbance of the scL-TMZ complex at 320 nm using a Beckman spectrophotometer. A standard curve of TMZ concentrations spanning 0.001 to 0.1 mM TMZ was also generated by measuring absorbance at 320 nM using DMSO as the blank. Free TMZ was separated from complexed scL-TMZ by filtration through a Vivaspin® 500, 5 kDa MWCO (GE Healthcare, UK). 200 ul of the diluted scL-TMZ complex was loaded onto the filter and centrifuged at 14,000 g for 15 min at room temperature. The flow through was collected and the volume (175 ul), and optical density at 320 nm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com