Methods for the Effective Treatment of Metastatic Cancer
a metastatic cancer and effective treatment technology, applied in the field of metastatic cancer effective treatment, can solve the problems of inability to obtain complete response (crs) in patients with most types of metastatic cancer, few drug regimens give high rates of complete response in patients with metastatic cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment e1
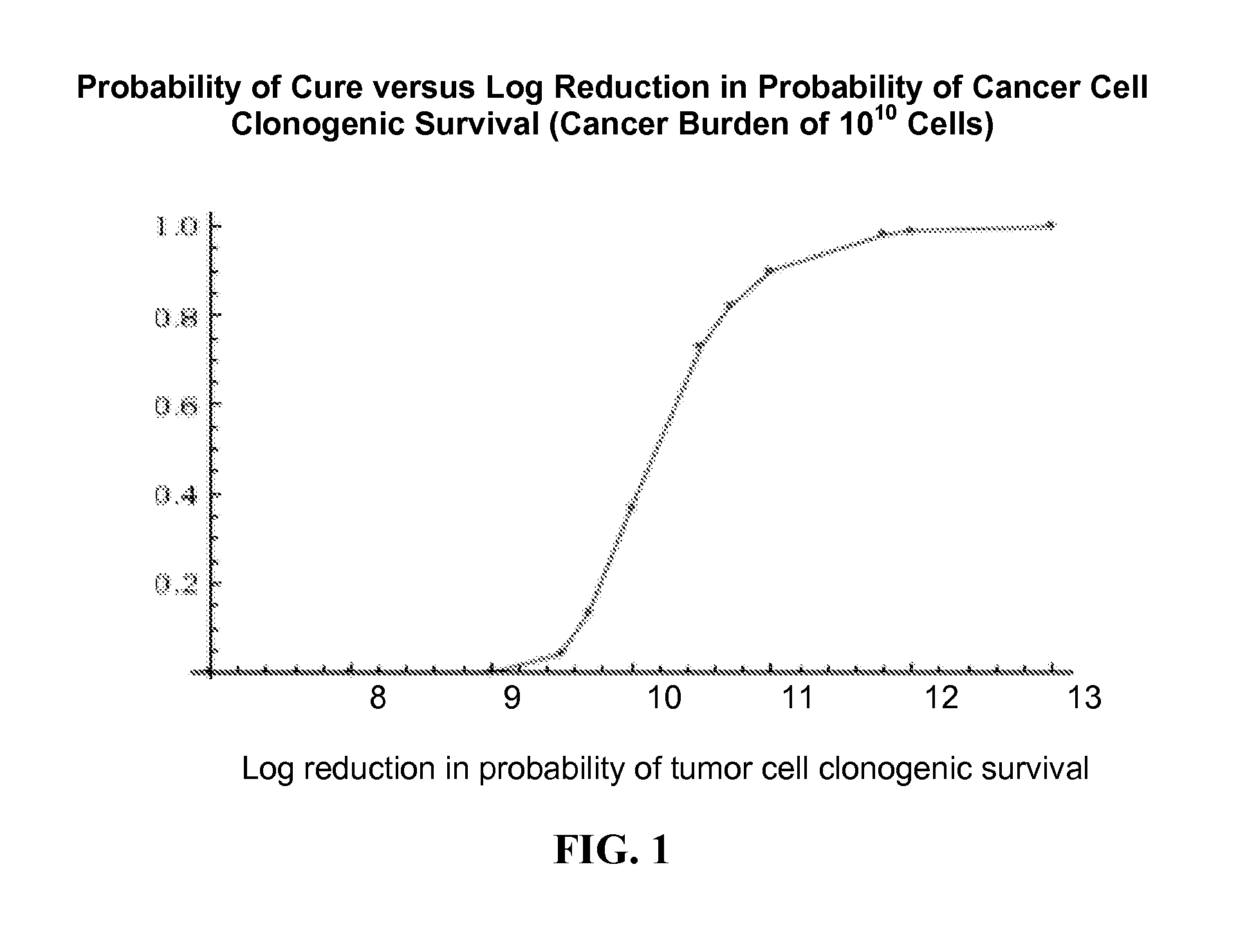
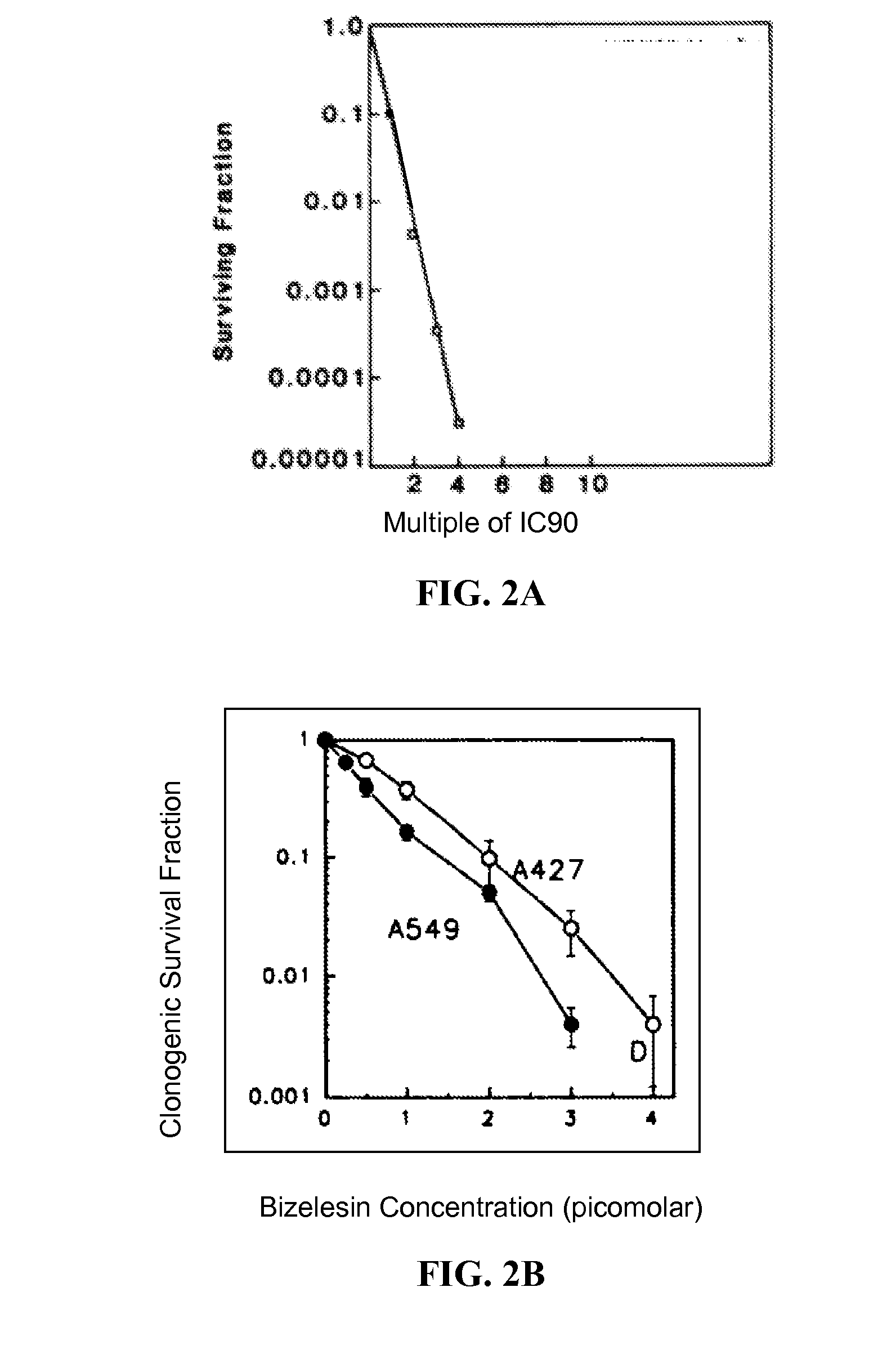
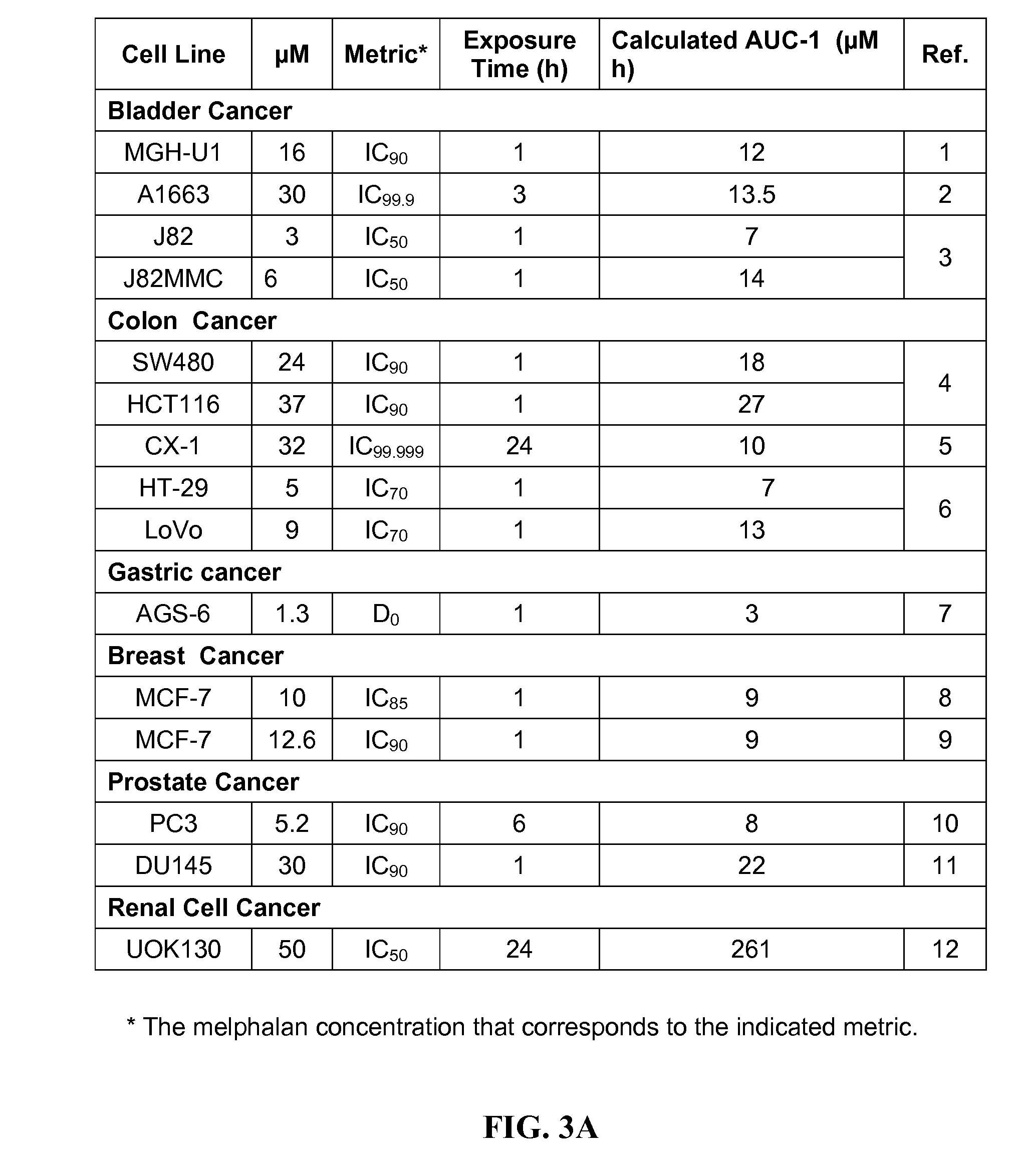
[0193]Embodiment E1 of the present invention is a method for obtaining CRs and durable, long-term CRs in patients with refractory metastatic cancers wherein the method is comprised of the administration of a set of one or more drugs that irreversibly inhibit the potential for cancer cell proliferation.
embodiment ee1
[0194]Embodiment Ee1 of the present invention is a set of drugs for use in a regimen for the treatment of refractory metastatic cancers and to obtain high rates of complete responses and durable, long-term complete responses in patients, wherein said set of drugs irreversibly inhibit the potential for cancer cell proliferation.
[0195]E1 can be expressed in an essentially equivalent form, as a method for obtaining high probabilities of a CR and a durable, long-term CR in a patient with refractory metastatic cancer, wherein said method is comprised of the administration of a set of one or more drugs that irreversibly and permanently inhibit the potential for cancer cell proliferation. Non-refractory metastatic cancers are generally sensitive to chemotherapy. While refractory metastatic cancers are generally resistant to chemotherapy. An effective treatment of refractory metastatic cancer would necessarily be an effective treatment for non-refractory metastatic cancer. The evolutionary ...
embodiment e2
[0196]Embodiment (E2) of the present invention is a method for the effective treatment of non-refractory metastatic cancer comprised of the administration of a set of one or more drugs, wherein said set of drugs irreversibly inhibits the potential for cancer cell proliferation and wherein said method gives improved patient outcome compared to current established therapies for the particular type of metastatic cancer.
Embodiment Ee2
[0197]Embodiment Ee2 of the present invention is a drug or set of drugs that irreversibly inhibit the potential for cancer cell proliferation for use in a regimen for the treatment of non-refractory metastatic cancers to achieve improved patient outcome compared to current established therapies for the particular type of metastatic cancer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com