Fully human anti-VEGF (Vascular Endothelial Growth Factor) monoclonal antibody and preparation method as well as application thereof
一种单克隆抗体、全人源的技术,应用在生物领域,能够解决亲和力不高、未能达到全人源化等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] Example Preparation of Antibody
[0020] (1) Cloning of human antibody light and heavy chain constant region genes
[0021] Lymphocyte separation fluid (product of Dingguo Biotechnology Development Co., Ltd.) was used to separate healthy human lymphocytes, and total RNA was extracted with Trizol reagent (product of Invitrogen Company). Research, 1982, 10: 4071-4079) respectively designed primers using RT-PCR reaction to amplify the antibody heavy chain and light chain constant region genes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector (promega company product). After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the heavy chain constant region (C H ) nucleotide sequence and amino acid sequence. SEQ ID NO: 3 and SEQ ID NO: 4 show the light chain constant region (C L ) nucleotide sequence and amino acid sequence. The correct clone in this...
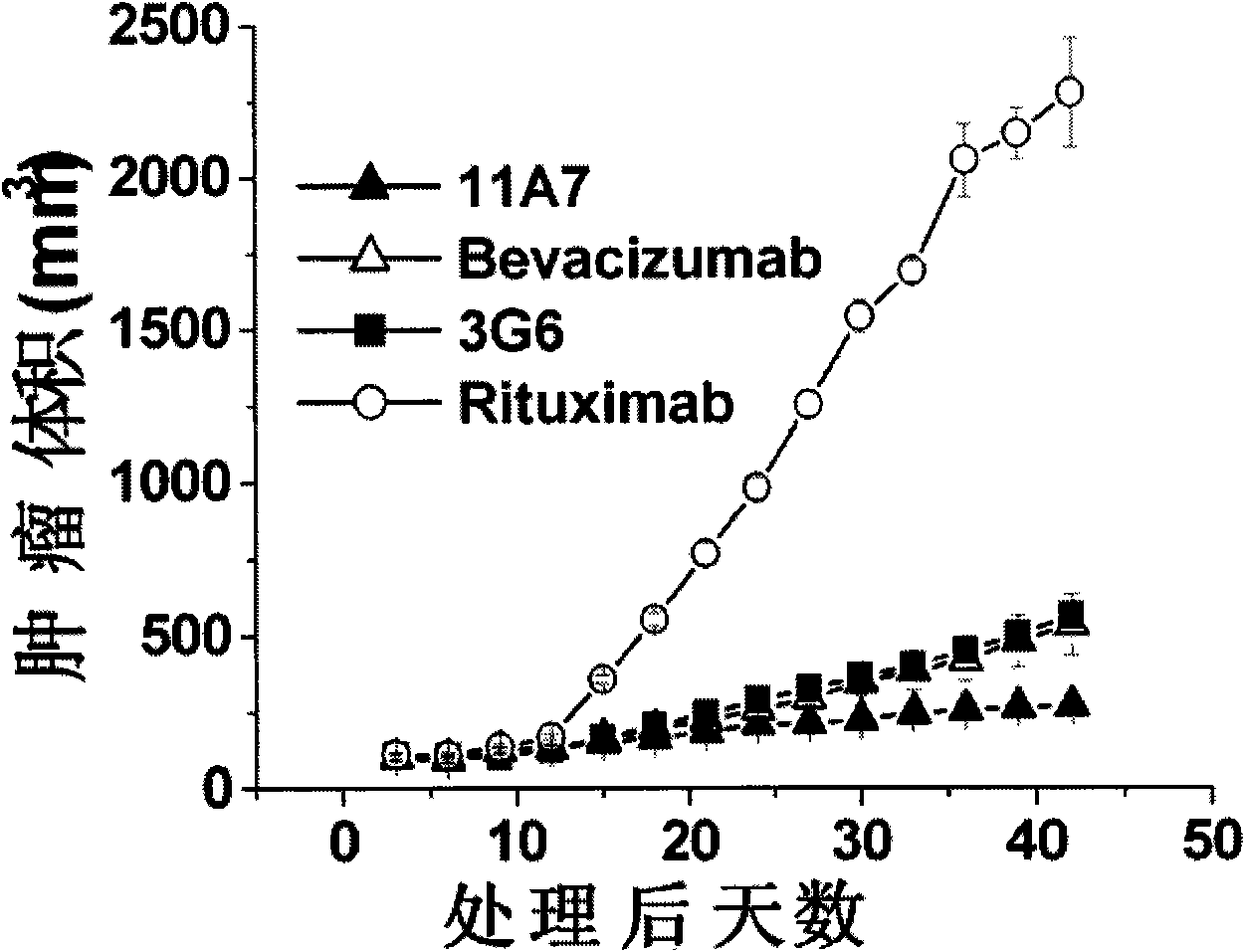
experiment example 1
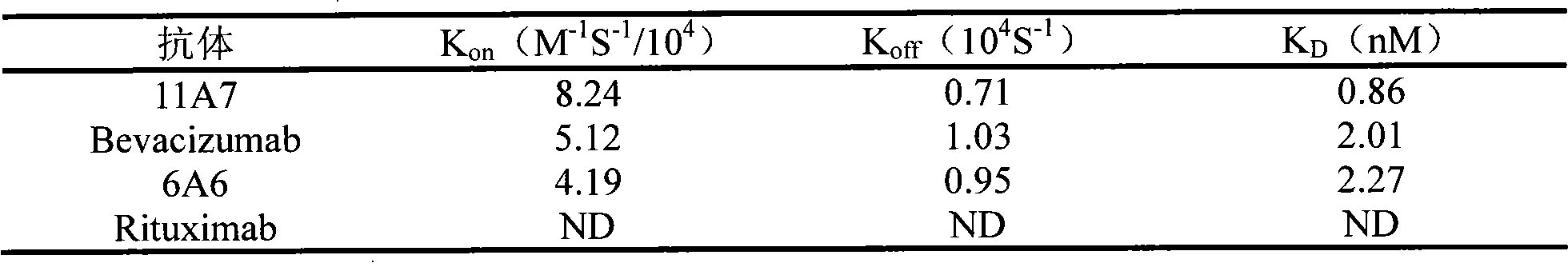
[0034] Experimental Example 1. VEGF Antibody Affinity Determination
[0035] The affinity constant of VEGF antibody was detected using Biacore T100 (Biacore AB, Uppsala, Sweden). VEGF165 (product of R&D Company) was covalently bonded to the CM5 biosensor chip (GE Healthcare) through amino groups, and the fully human antibody 11A7, Bevacizumab, and human antibody 6A6 (according to Chinese patent application number 02111093.X application date 2002 Prepared by the method disclosed in the invention title "Humanized anti-vascular endothelial growth factor monoclonal antibody and its preparation method and pharmaceutical composition" on February 30) and negative control antibody (Rituximab commercially available product) (in PBS / 0.05% TWEEN-20 (ICIAmericas) (detergent) solution was made into different concentrations (2-fold ratio concentration dilution) and passed through the chip at a flow rate of 50 μl / min. After each check, residual antibody was eluted from the immobilized ligan...
experiment example 2
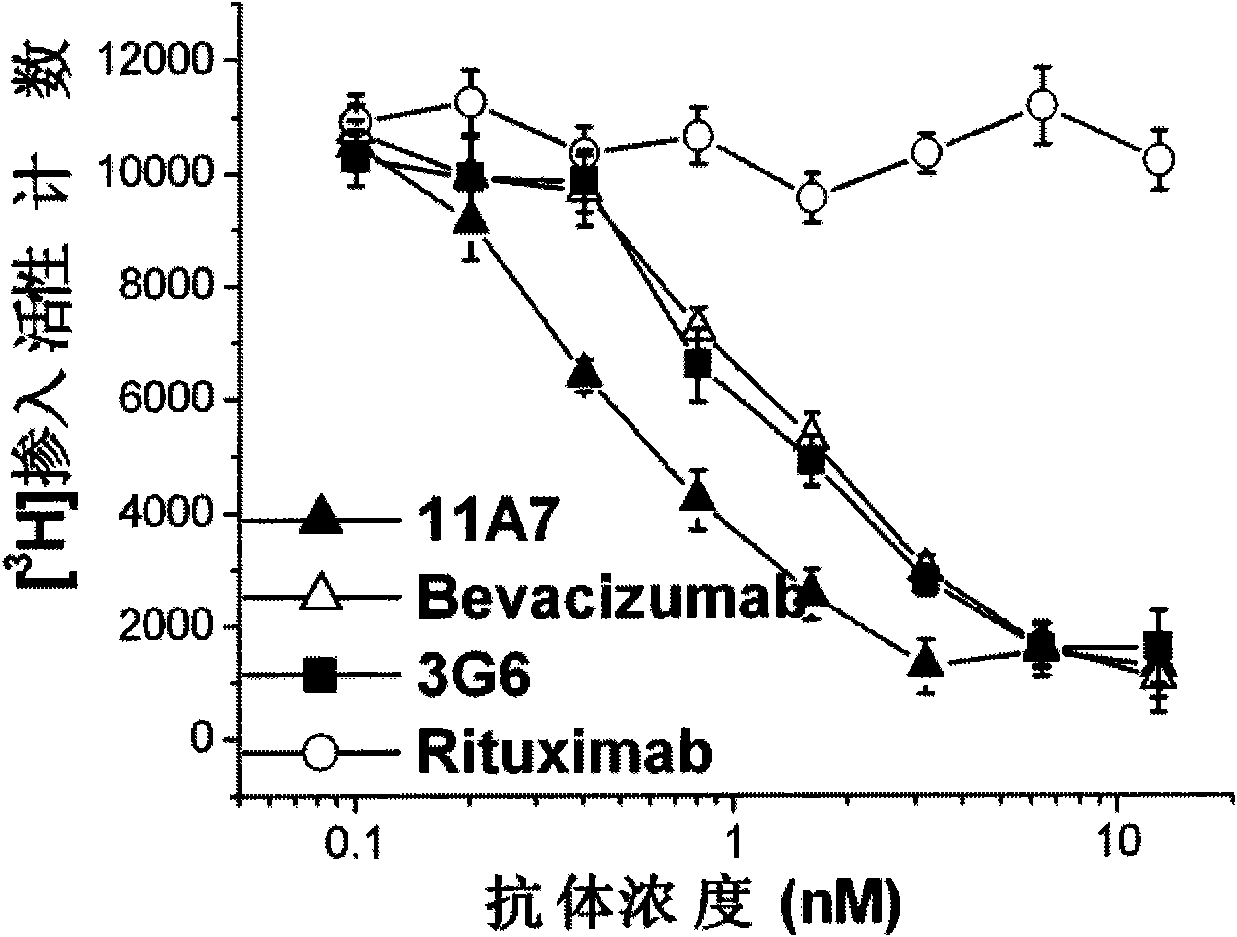
[0038] Experimental example 2. VEGF antibody inhibits HUVEC cell proliferation experiment
[0039] Experimental procedure: take HUVEC cells (Cascade Biologics) in a good growth state, and adjust the cell concentration to 2.5×10 4 / ml, seeded in 96-well cell culture plate, 200μl / well, at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, replace the serum-free medium, continue culturing for 72 hours, add VEGF antibodies with different concentration gradients, and use the anti-CD20 antibody Rituximab as a negative control, take 3 parallel wells for each concentration, incubate at 37°C for 1 hour, add the final concentration 25ng / ml of VEGF165 (R&D) was cultured for 24 hours, then 10 μl [3H].TdR (18.5 kBq / well) was added, and incubated in a 37°C incubator for 7 hours. 3H] liquid scintillation counter for determination. Such as figure 1 shown.
[0040] The results showed that the negative control antibody (Rituximab) could not effectively inhibit VEGF-induced HUVEC c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com