A combined ligand, combined biomimetic chromatography medium and its preparation method and application
A combined biomimetic layer technology, applied in the field of biomimetic chromatography, can solve problems such as single ligand structure, low antibody selectivity, and antibody difficulty, and achieve the goal of improving elution conditions, strengthening hydrophobic interaction, and reducing elution difficulty Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
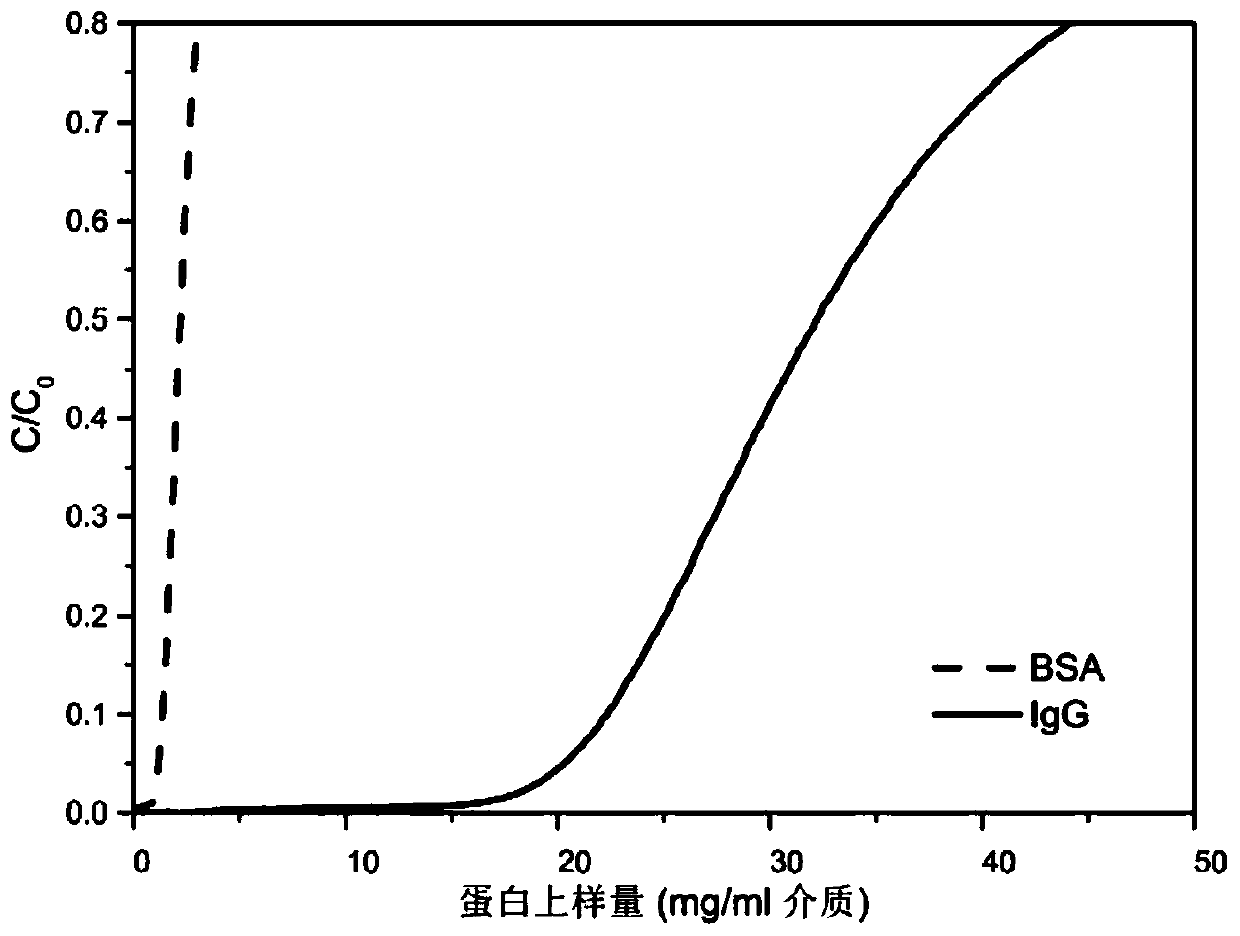
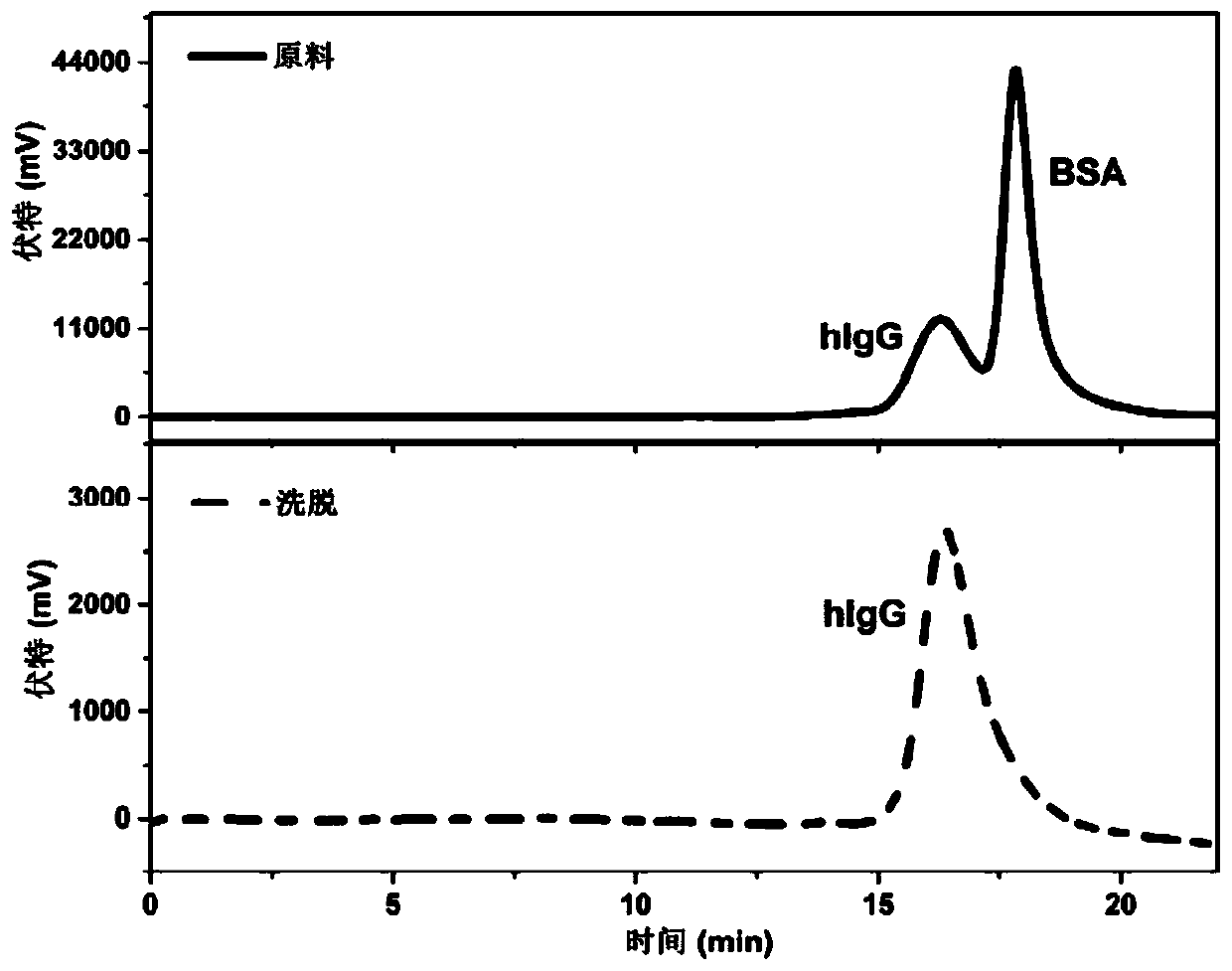
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of combined ligand
[0055] With the help of computer molecular simulation, analyze and evaluate the key residues of protein A and antibody Fc binding site, screen and design the combinatorial ligand of tripeptide-heterocyclic small molecule, whose sequence is phenylalanine-tyramine Acid-glutamic acid-5-aminobenzimidazole.
[0056] Combined ligand, the structural formula is as follows:
[0057]
[0058] The combinatorial ligand can be synthesized by the chemical synthesis method in the prior art, and the combinatorial ligand in this example is entrusted to China Peptide Biochemical Co., Ltd. to prepare it.
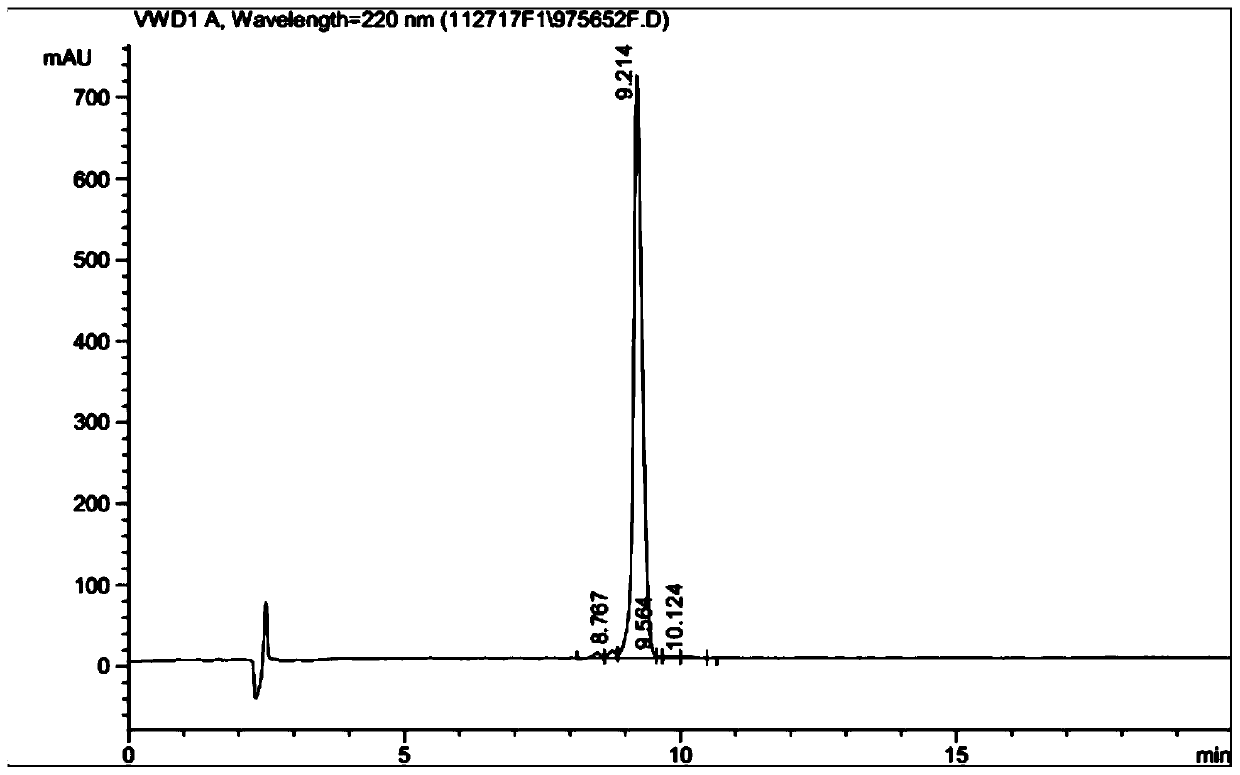
[0059] Carry out high performance liquid chromatography characterization for the combined ligand in embodiment 1, such as figure 1 shown.
Embodiment 2
[0060] Example 2: Preparation of Combined Biomimetic Chromatography Medium
[0061] Take 5.0 g of drained agarose gel, add 5.0 g of 20% (v / v) dimethyl sulfoxide, 2.5 g of allyl bromide and 1.0 g of sodium hydroxide, activate it on a shaker at 150 rpm at 30 ° C for 24 hours, and pump Filter and wash with deionized water to obtain an activated chromatography matrix.
[0062] Mix the activated chromatographic matrix, 10.0g 50% (v / v) acetone and 1.5g N-bromosuccinimide for bromoalcoholization, react on a shaking table at 150rpm at 30°C for 3h, filter with suction, and use deionized Washing with water yields a bromoalcoholated substrate.
[0063] Mix 2.5g dimethyl sulfoxide and 5.0g 1M sodium carbonate buffer, add 0.5g phenylalanine-tyrosine-glutamic acid-5-aminobenzimidazolidine, fully dissolve, then add bromoalcohol The chromatographic matrix was reacted on a shaking table at 150 rpm at 30°C for 12 hours, and was repeatedly washed with deionized water, 0.1M HCl, and 0.1M NaOH t...
Embodiment 3
[0067] Example 3: Preparation of Combined Biomimetic Chromatography Medium
[0068] Take 5.0 g of drained agarose gel, add 2.5 g of 20% (v / v) dimethyl sulfoxide, 0.5 g of allyl bromide and 0.5 g of sodium hydroxide, activate it on a shaker at 150 rpm at 30 ° C for 24 hours, and pump Filter and wash with deionized water to obtain an activated chromatography matrix.
[0069] Mix the activated chromatographic matrix, 5.0g 50% (v / v) acetone and 0.5g N-bromosuccinimide for bromoalcoholization, react on a shaking table at 150rpm at 30°C for 1h, filter with suction, and use deionized Washing with water yields a bromoalcoholated substrate.
[0070] Mix 3.0g dimethyl sulfoxide and 5.0g 1M sodium carbonate buffer, add 0.5g phenylalanine-tyrosine-glutamic acid-5-aminobenzimidazolidine, fully dissolve, then add bromoalcohol The chromatographic matrix was reacted on a shaking table at 150 rpm at 30°C for 8 hours, and was repeatedly washed with deionized water, 0.1M HCl, and 0.1M NaOH to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com