Anti-il-1β humanized monoclonal antibody and preparation method and application thereof
An antibody and variable region technology, applied in the field of anti-IL-1β humanized monoclonal antibody and its preparation, can solve the problems of short half-life, strong immunogenicity, and low specificity, and achieve improved affinity and strong IL Effect of -1β neutralizing ability and high antibody affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
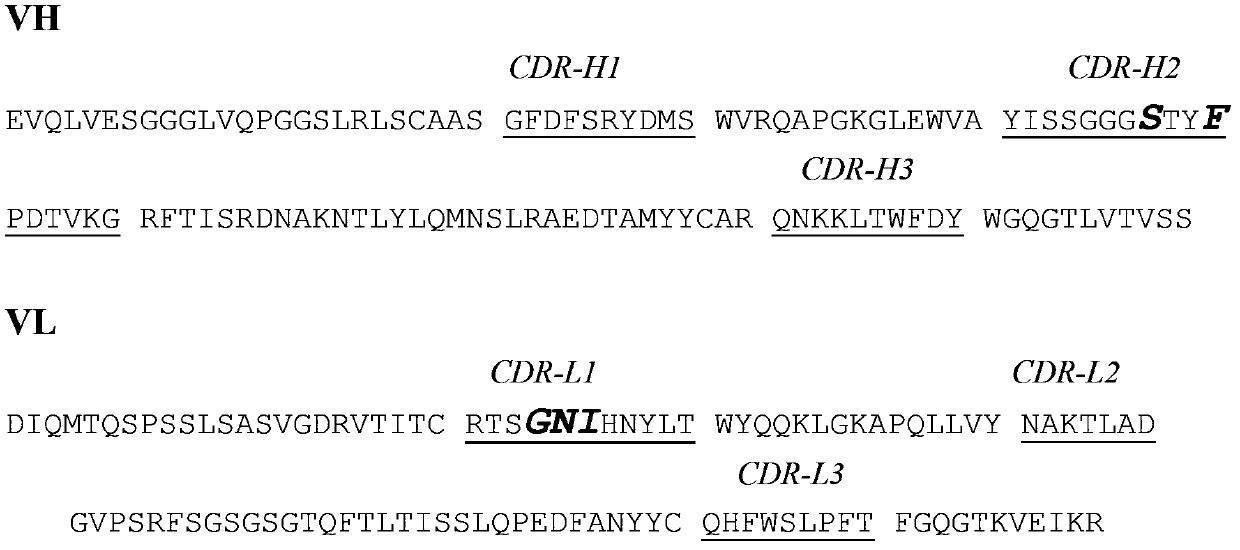
[0035] Example 1 Template single-chain antibody gene synthesis and CDR mutant phage library construction
[0036] The sequences of the heavy chain variable region (VH) and light chain variable region (VL) of the template antibody are as follows figure 1 As shown, the GGGGSGGGGSGGGGS polypeptide sequence is linked between VH and VL to form a single-chain antibody scFv, and the corresponding DNA sequence can be obtained by full-length gene synthesis. Perform detailed CDR site analysis on the VH and VL sequences of the template antibody, and design random mutation primers for CDR-H2, CDR-H3, CDR-L1, and CDR-L3 regions accordingly. Using mutation primers to do PCR synthesis of VH and VL gene fragments containing mutations, and then performing PCR splicing into new mutant scFv single-chain antibody genes. The mutated scFv gene was digested and ligated into the phage display vector pCANTAB5E (GE Healthcare Life Sciences), which was then used to transform TG1 Escherichia coli cells ...
Embodiment 2
[0037] Example 2 Affinity-based screening of phage antibody mutant library
[0038] The constructed phage mutant library was used to pan against IL-1β antigen. Phage panning was performed in liquid phase using biotinylated antigen. At lower and lower antigen concentrations, only phages displaying high-affinity antibodies will bind to the antigen, which can then be called out and retained using streptavidin magnetic beads. After several times of panning, the library was sampled and sequenced. There was no significant enrichment of new variants in the CDR-H3 and CDR-L3 libraries, while most clones in the CDR-H2 and CDR-L1 libraries had been converted to new variants. variant.
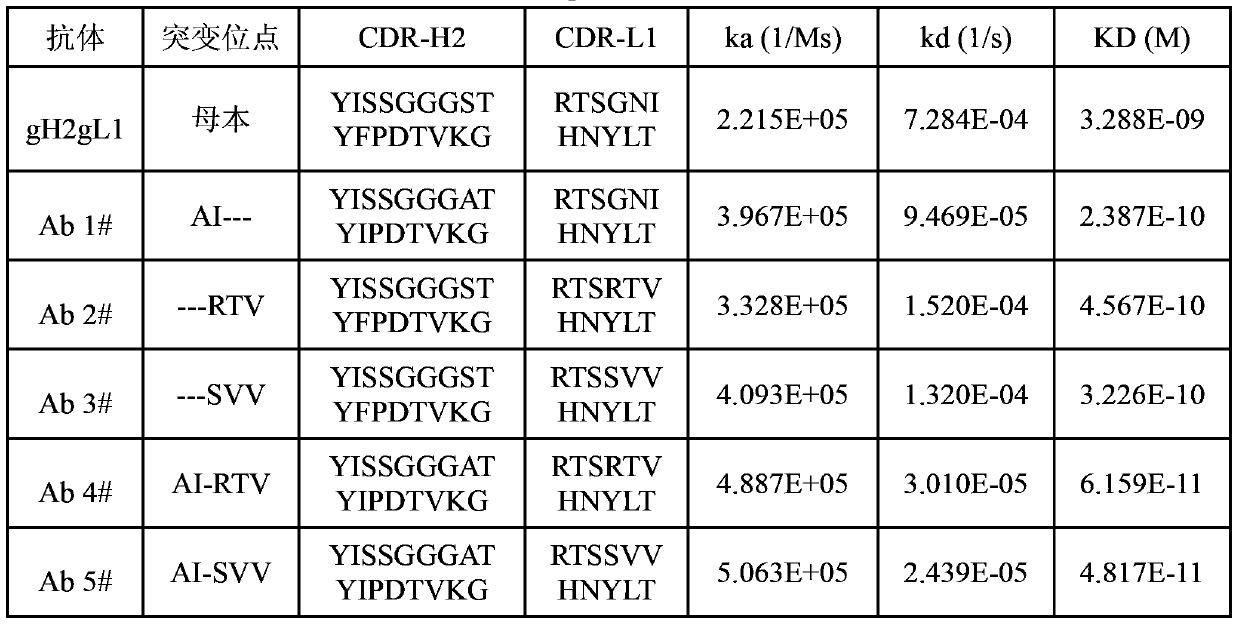
[0039] 50 different clones were selected from the CDR-H2 and CDR-L1 libraries after panning, and scFv proteins were induced to express and purified. Then use BiacoreT200 to quickly detect its dissociation curve with the antigen and compare the Off-rate. Among them, clones that dissociate slowly from t...
Embodiment 3
[0040] Example 3 Expression, purification and affinity constant determination of full-length antibody IgG in eukaryotic cells
[0041] Using the obtained single-chain antibody gene containing the "AI" mutation as a template, the human antibody heavy chain gene was spliced with the human full-length antibody IgG heavy chain constant region by overlapping PCR, and then digested with HindIII and EcoRI restriction enzymes Afterwards, it was purified and recovered by agarose gel electrophoresis, and then ligated into the pcDNA3.1(+) plasmid (Invitrogen Company) to construct the human heavy chain eukaryotic expression vector pcDNA3.1(+)(VHCH). Similarly, using the single-chain antibody gene containing the "SVV" or "RTV" mutation as a template, the human antibody light chain gene was spliced with the human full-length antibody IgG light chain constant region by overlapping PCR, and then HindIII and EcoR I were used to After digestion with restriction enzymes, it was purified and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com