Method for treating atrophic age related macular degeneration
A macular degeneration and dryness technology, applied in the directions of pharmaceutical formulations, organic active ingredients, medical preparations with non-active ingredients, etc., can solve the problem that AMD has no effective treatment and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
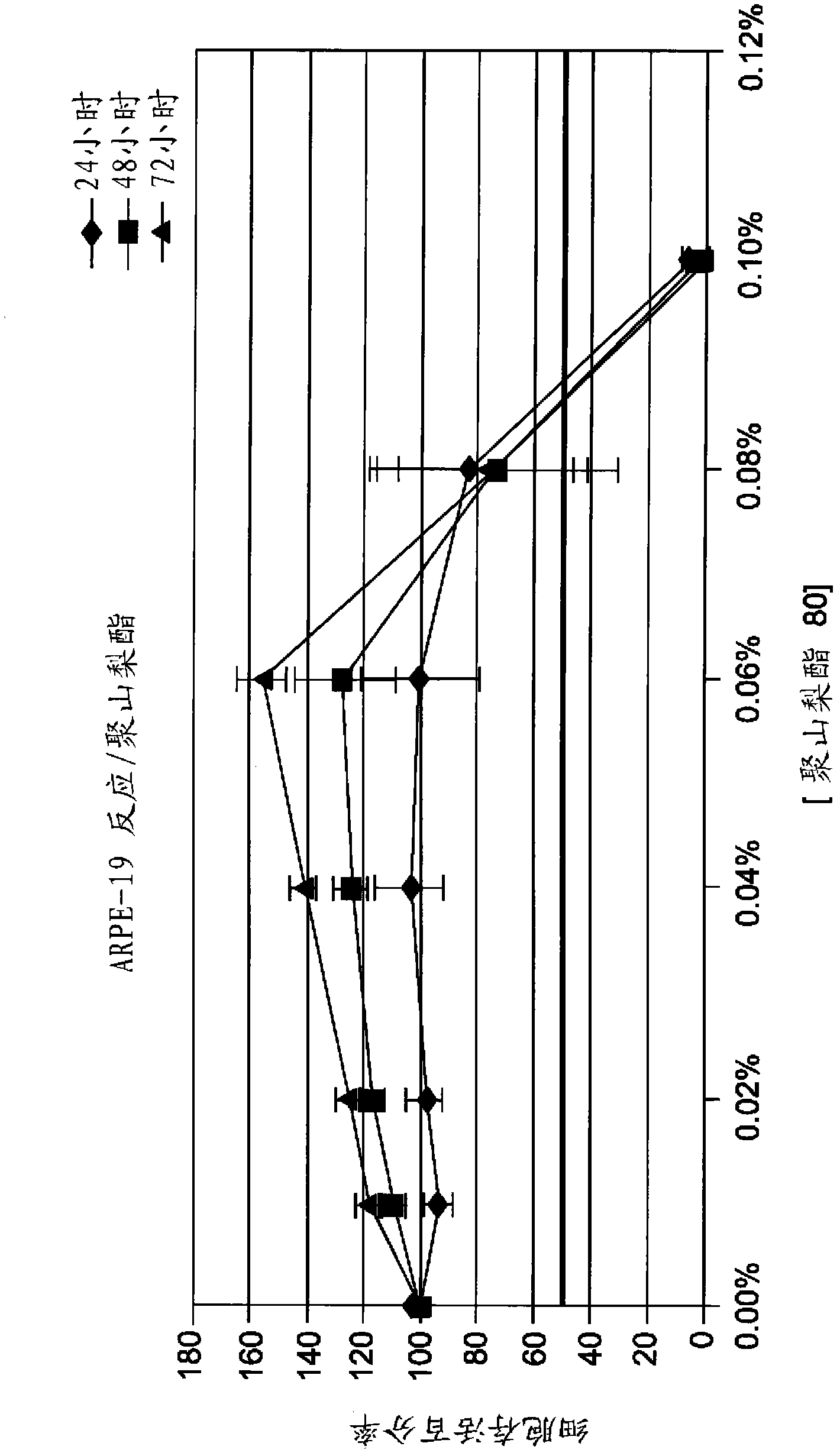
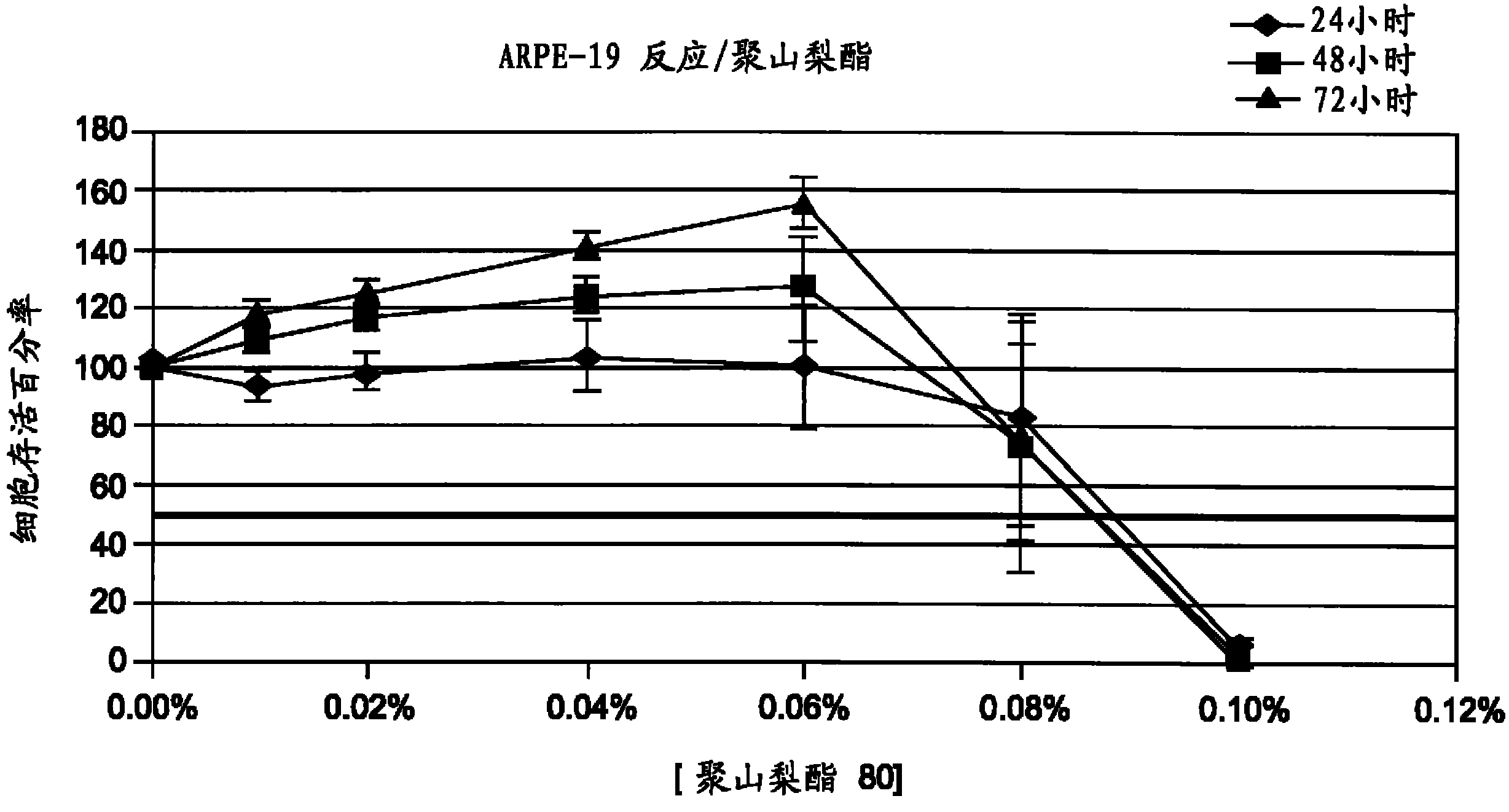
Image
Examples
Embodiment 1
[0103] Intravitreal bevacizumab-PLGA microspheres for the treatment of dry AMD
[0104] A 78-year-old man presented with age-related macular degeneration and cataracts in both eyes. The patient also had a history of cardiovascular disease and an inferior myocardial infarction 6 months earlier. The patient complained of blurred vision and metamorphopsia in the right eye. On examination, the visual acuity was 20 / 400 in the right eye and 20 / 32 in the left eye. Retinal examination revealed subfoveal choroidal neovascularization (CNV) in the right eye (wet AMD in the right eye), approximately 1 optic disc area in size with peripheral hemorrhage and edema. The opposite left eye showed high-risk features for developing wet AMD, such as drusen with soft amorphous appearance in the fovea but no evidence of choroidal neovascularization, and was confirmed by fluorescein angiography (left eye dry AMD) . The patient started with monthly intravitreal injections of bevacizumab (an anti-...
Embodiment 2
[0111] Low-dose intravitreal bevacizumab-PLGA microspheres for the treatment of dry AMD
[0112] A 74-year-old male was diagnosed with age-related AMD in one (right) eye and wet AMD in the other (left) eye. His right eye vision was 20 / 40. He was treated by intravitreal injection of a sustained release drug delivery system into a dry AMD eye. The sustained release drug delivery system contained a total of about 6 micrograms (low dose) of the active agent bevacizumab in a polymeric carrier. The polymer carrier is high viscosity hyaluronic acid or PLGA or PLA, which is combined with the bevacizumab anti-neovascular agent to form a plurality of microspheres or a single integrated implant in which the bevacizumab is uniformly distributed . Alternatively, the sustained-release drug delivery system may comprise bevacizumab microspheres or implants in hyaluronic acid (cross-linked or non-cross-linked) such that there is simultaneous viscous polymerization in the same drug deliver...
Embodiment 3
[0114] Intravitreal ranibizumab-PLGA microspheres for the treatment of dry AMD
[0115] An 83-year-old woman woke up with blurred vision in her left eye. She has a history of glaucoma, s / p cataract removal with IOL (intraocular lens), and dry AMD in both eyes, and is taking Alphagan P eye drops. The patient was examined by her ophthalmologist, who was diagnosed with wet AMD in the left eye and was immediately sent to a retinal specialist. Vision is 20 / 25 in the right eye and 20 / 200 in the left eye. Retinal examination revealed dry changes in the macula of the right eye, but high-risk features such as large drusen and numerous pigmented changes in the subfoveal area. The macula of the left eye showed subfoveal CNV, approximately 2 discs in size, with surrounding macular edema and intraretinal hemorrhage. Fluorescein angiography confirmed the presence of a very typical looking left eye CNV. Monthly intravitreal injections of ranibizumab into this patient's wet AMD left eye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com