Methods of treating pterygium
a technology of pterygium and keloid, applied in the field of eye and skin conditions, can solve the problems of high recurrence rate of surgical management of pterygium and negatively affect the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
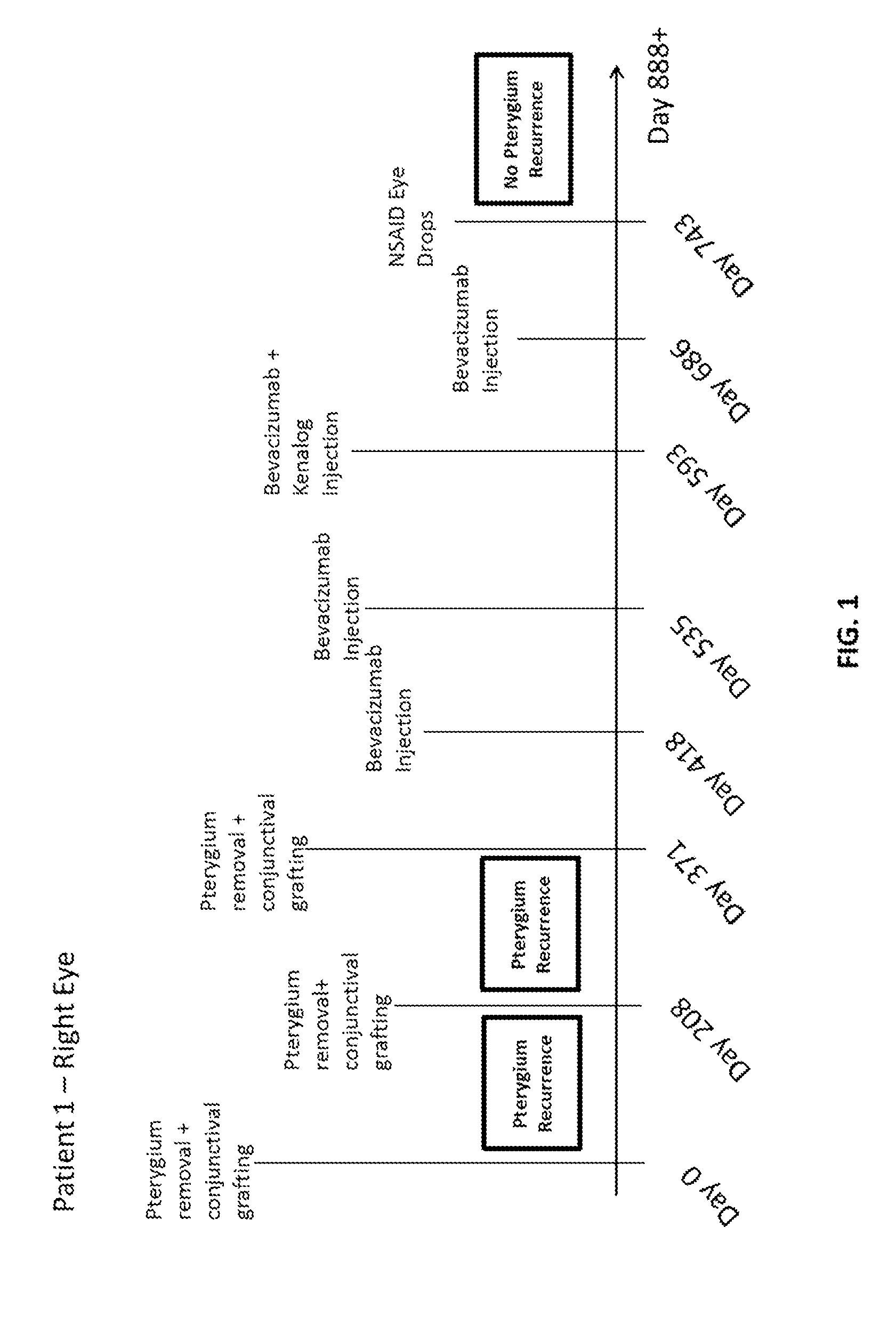
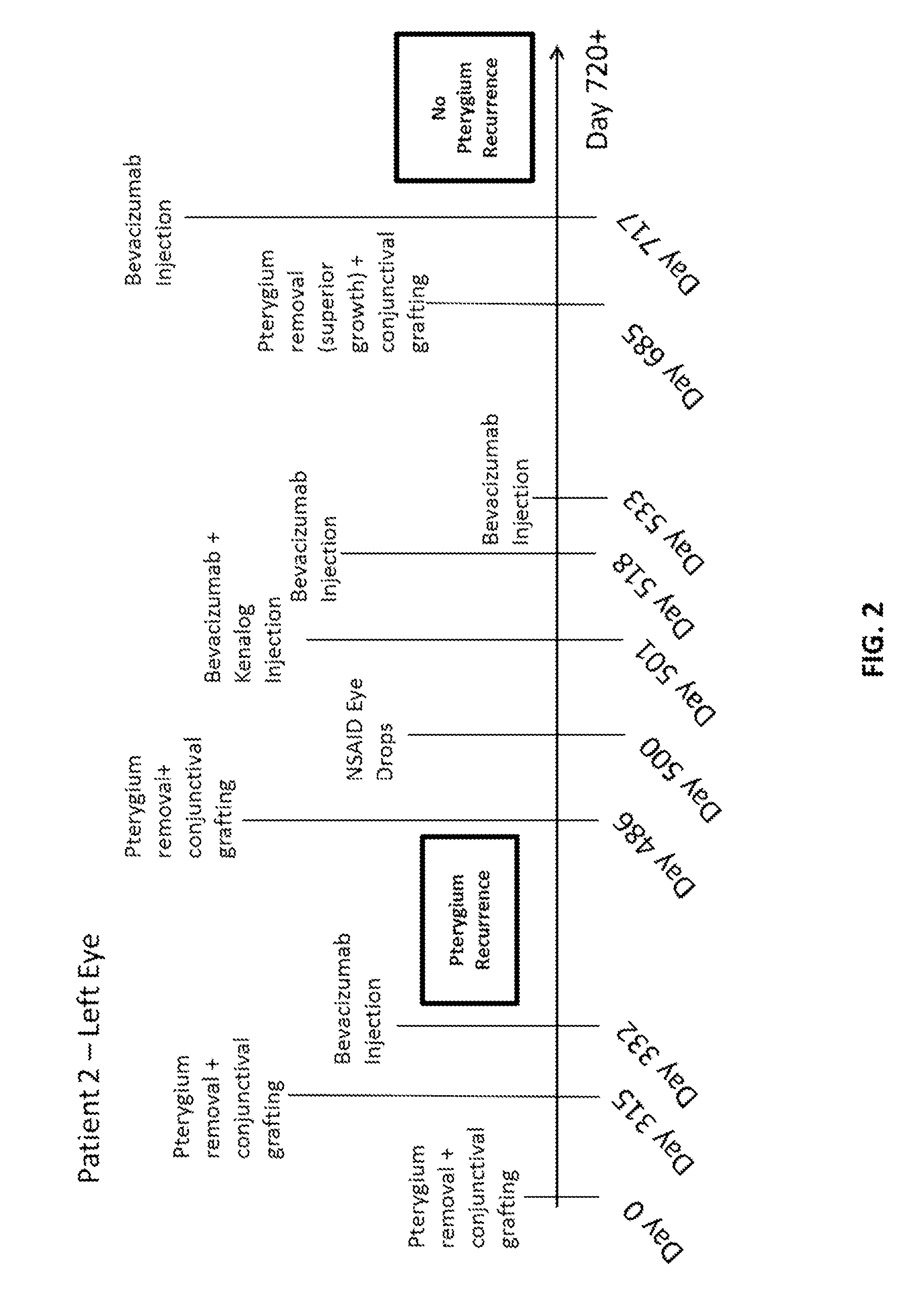
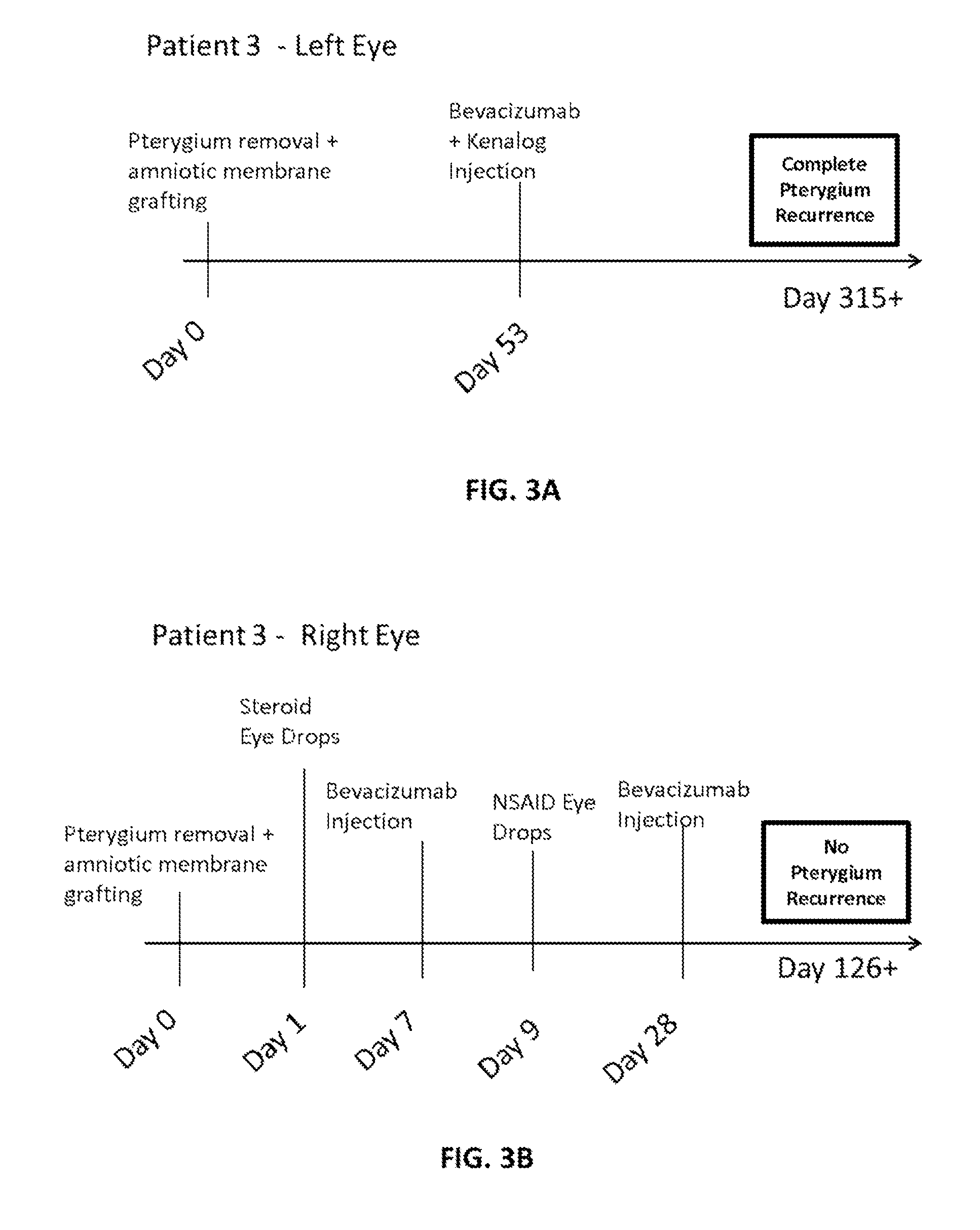
[0063]A topical anesthetic (proparacaine 0.5% eye drops or Alcaine manufactured by Alcon Laboratories Inc.) was applied to the eye following recent pterygiectomy (from ˜5 days to 2 months following surgery). The patients were examined to identify vascular growth on eye conjunctiva. A syringe containing bevacizumab (Avastin®) (Genentech / Roche) at a mixture of 2.5 mg / 0.1 mL was injected into the conjunctiva, and the plunger was drawn back to determine if blood was present in the syringe hub. If blood was present (indicating presence of blood vessel), the syringe needle was withdrawn. Bevacizumab was injected into areas adjacent to vascular growth, where blood vessels were dilated and numerous. If bleeding occurred (e.g., in the form of subconjunctival or subcutaneous hematoma), tamponade with Q-tips® was performed until bleeding stopped; then injection was resumed. In some cases, patients were injected with a combination of bevacizumab and Kenalo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com