Compound monoammonium glycyrrhizate S injection pharmaceutical composition as well as preparation method and application thereof
A technology of monoammonium glycyrrhizinate and monoammonium glycyrrhizinate, which is applied in the field of medicine, can solve the problems of difficult discovery of insoluble particles in medicines, affect human health, and poor stability, and achieve significant curative effect, improved stability, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0029] Monoammonium glycyrrhizinate S 520g, cysteine hydrochloride 390g, glycine 5.2kg, anhydrous sodium sulfite 520g, edetate disodium 52g, sodium chloride 2.08kg, activated carbon 130g, and finally dilute to 260L with water for injection.
[0030] The preparation method of this compound monoammonium glycyrrhizinate S injection pharmaceutical composition comprises the following steps:
[0031] S1. Weighing: Transfer monoammonium glycyrrhizinate S, cysteine hydrochloride, glycine, anhydrous sodium sulfite, disodium edetate and sodium chloride from the material temporary storage room to the weighing room for weighing ingredients; activated carbon It is necessary to carry out the carbon weighing and carbon dissolving treatment in the carbon storage and weighing chamber first, that is, weigh the medicinal charcoal with a weighing ...
Embodiment 2
[0037] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0038] Monoammonium glycyrrhizinate S 400g, cysteine hydrochloride 300g, glycine 4.0kg, anhydrous sodium sulfite 400g, edetate disodium 40g, sodium chloride 1.0kg, activated carbon 100g, and finally dilute to 180L with water for injection.
[0039] The preparation method of this compound monoammonium glycyrrhizinate S injection pharmaceutical composition is the same as in Example 1.
Embodiment 3
[0041] A compound monoammonium glycyrrhizinate S injection pharmaceutical composition, comprising the following raw materials in parts by weight:
[0042] Monoammonium glycyrrhizinate S 600g, cysteine hydrochloride 500g, glycine 6.0kg, anhydrous sodium sulfite 650g, edetate disodium 60g, sodium chloride 3.0kg, activated carbon 200g, and finally dilute to 450L with water for injection.
[0043] The preparation method of this compound monoammonium glycyrrhizinate S injection pharmaceutical composition is the same as in Example 1.
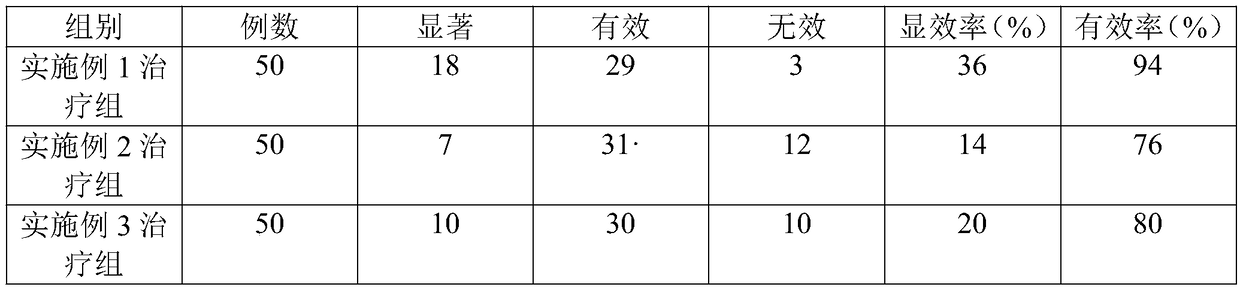
[0044] Drug efficacy verification:
[0045] In order to further illustrate the application value of the compound monoammonium glycyrrhizinate S injection pharmaceutical composition of the present invention, the stability and drug efficacy of the medicament in each embodiment of the present invention are verified, and the verification results are as follows:
[0046] 1. Stability experiment:
[0047] The medicaments prepared according to the formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com