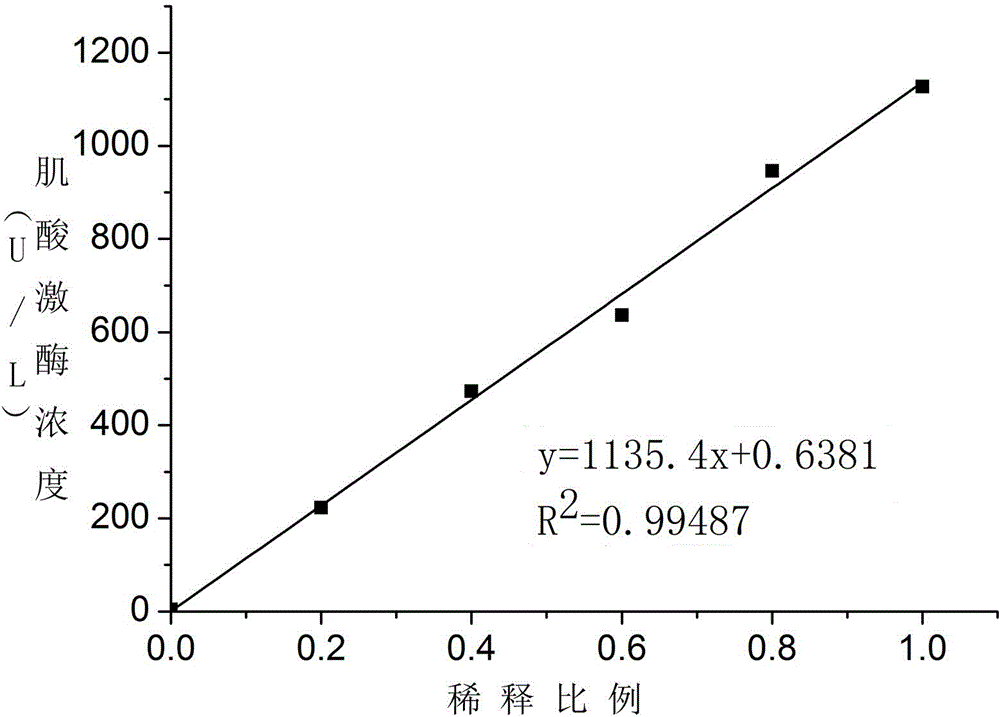
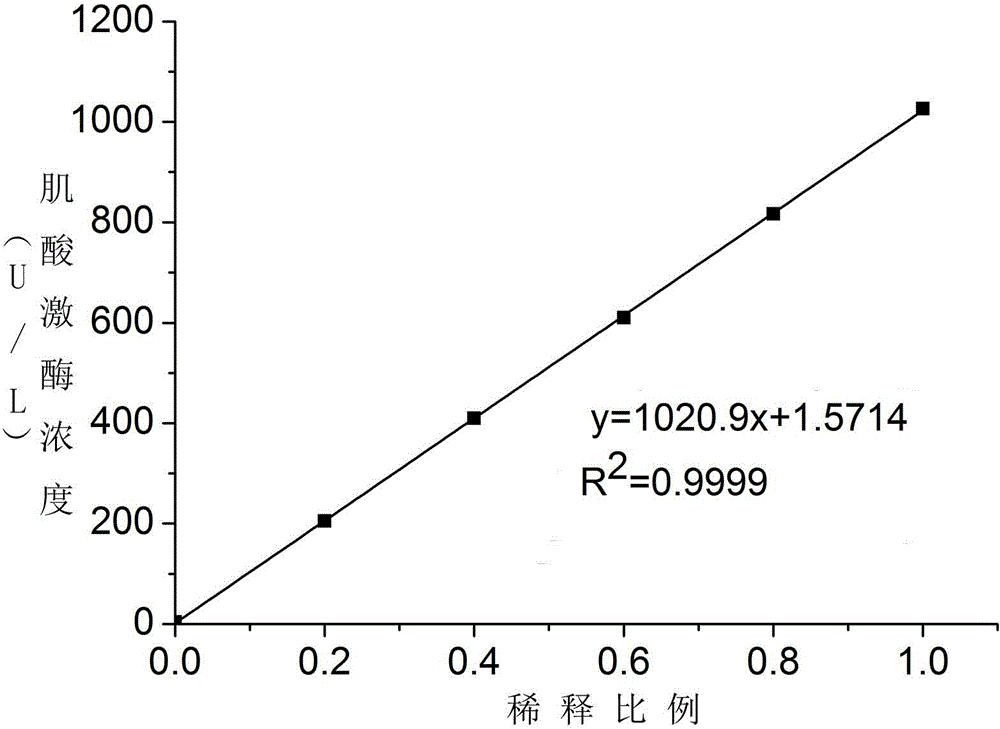
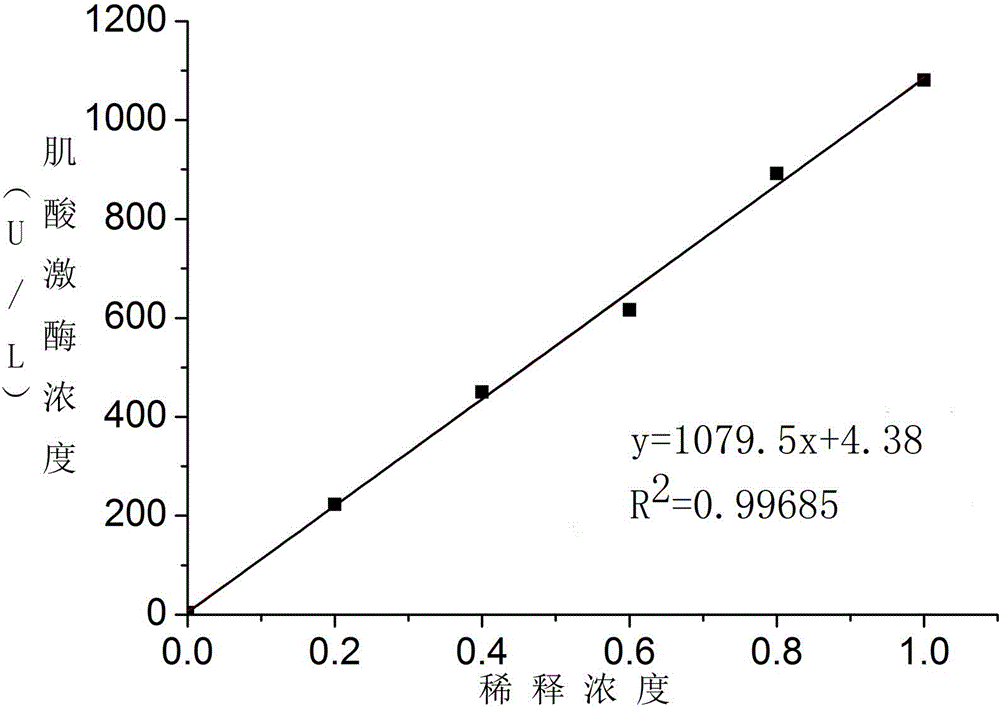
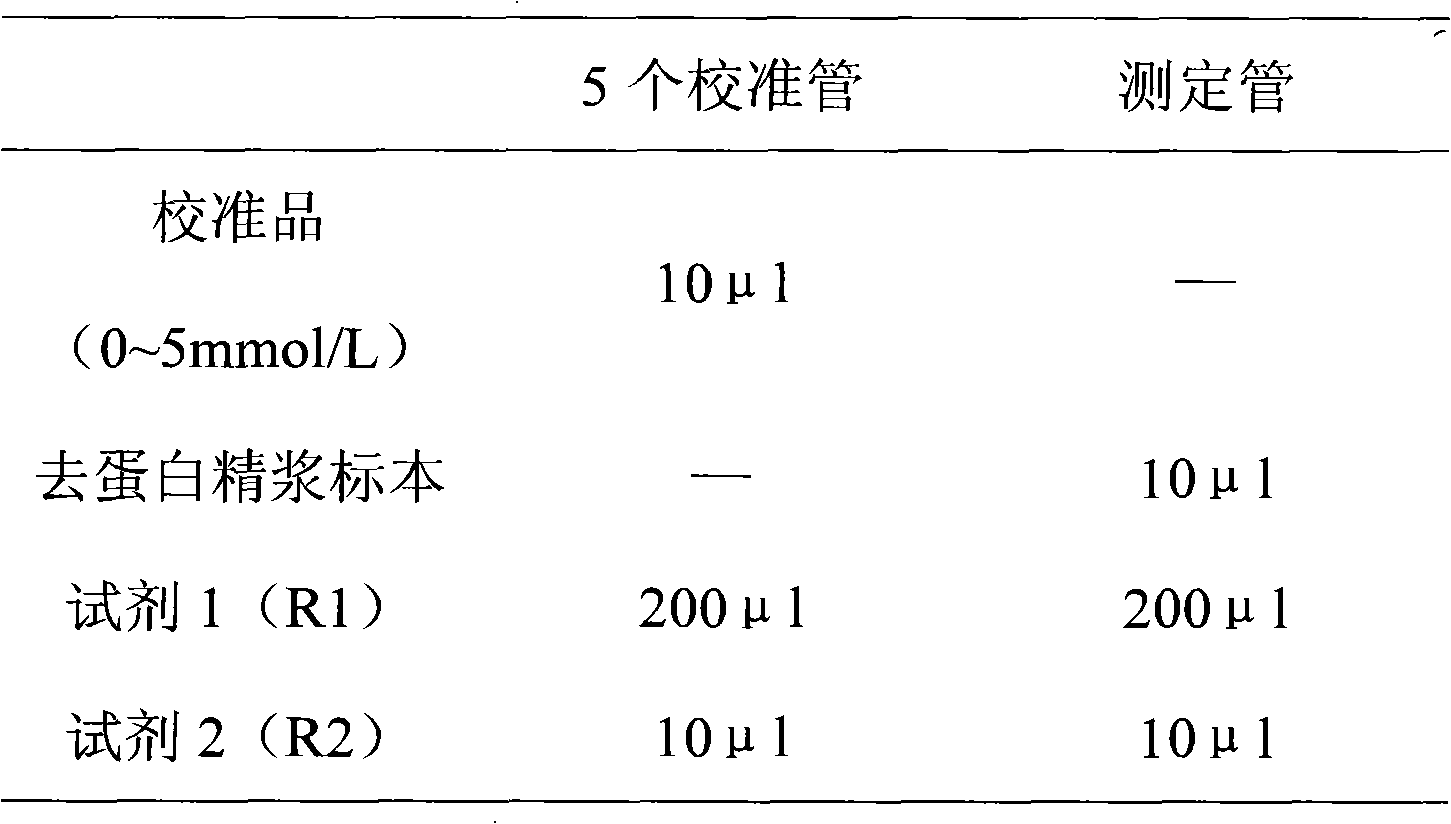
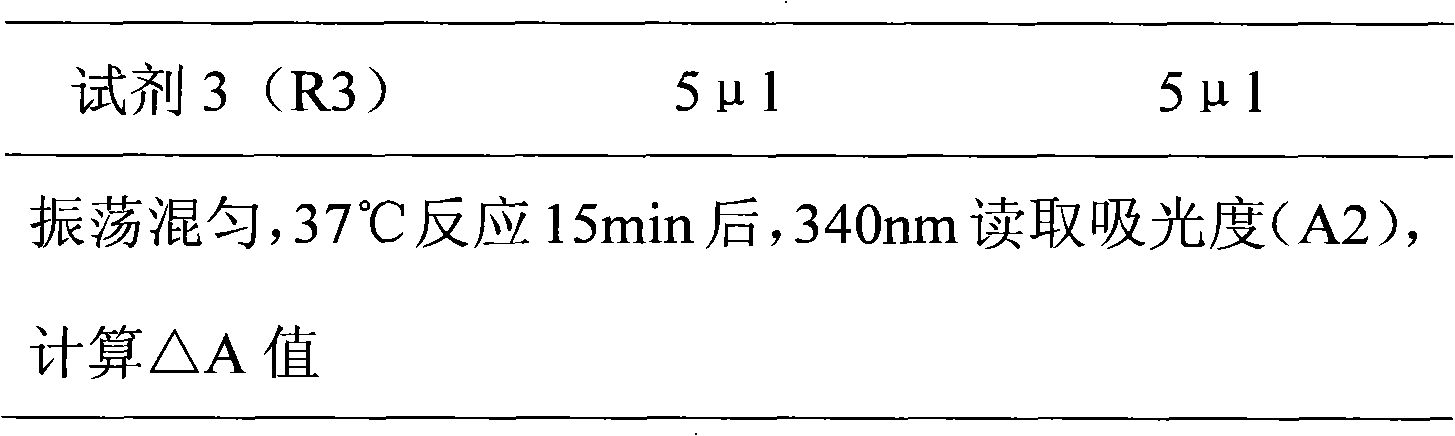
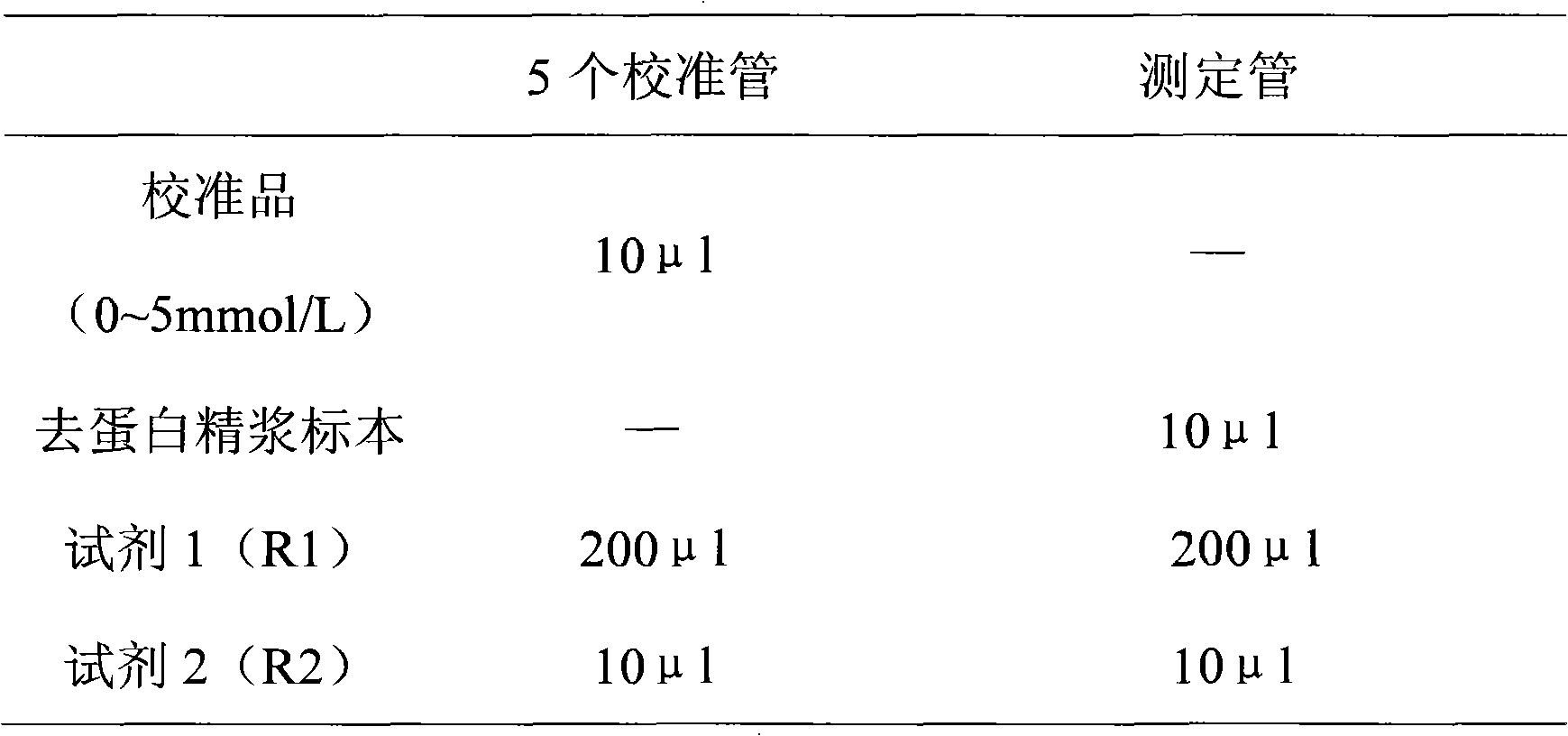
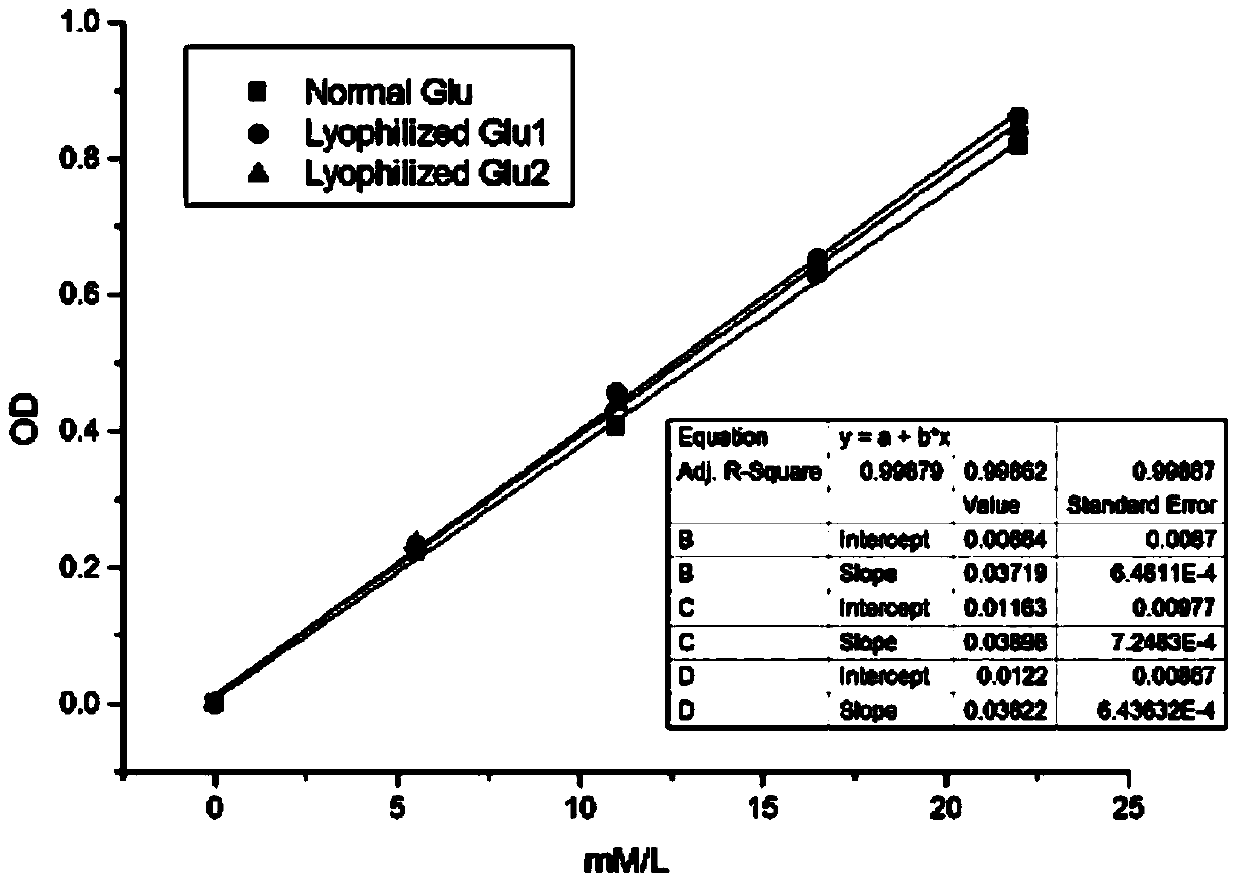
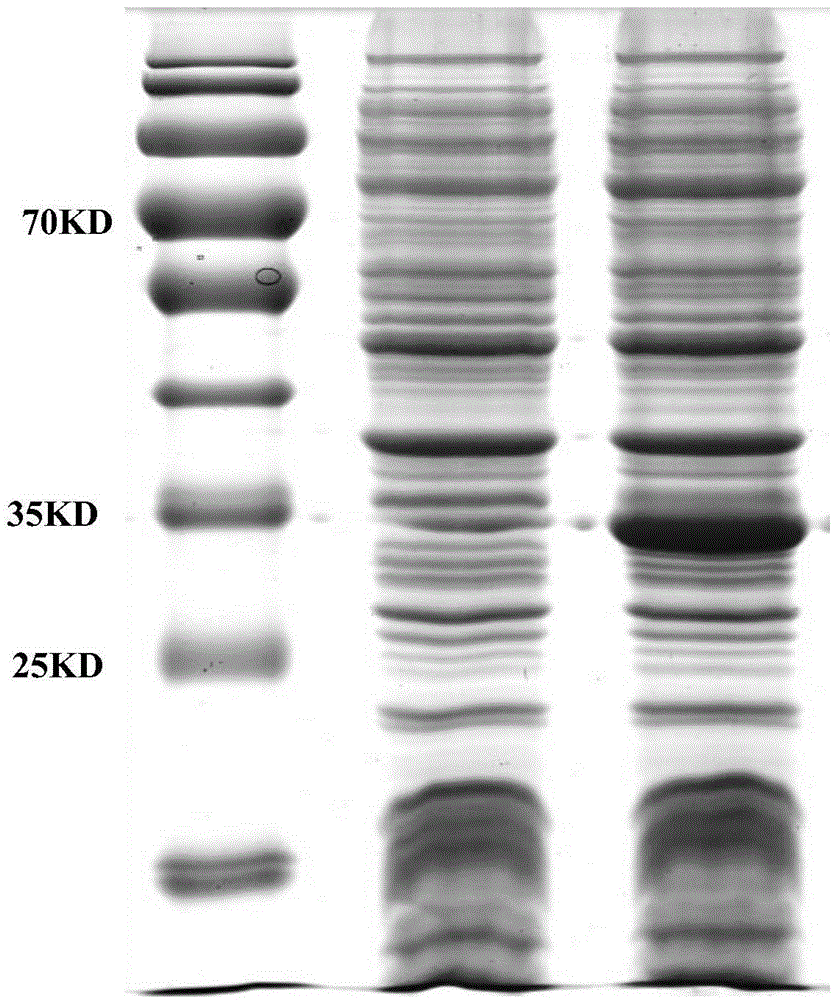
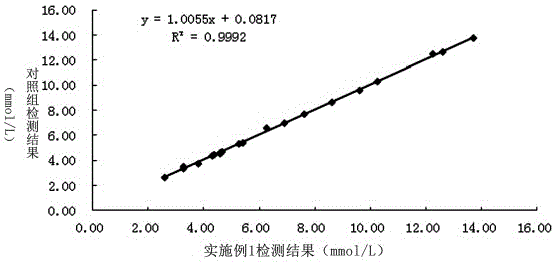
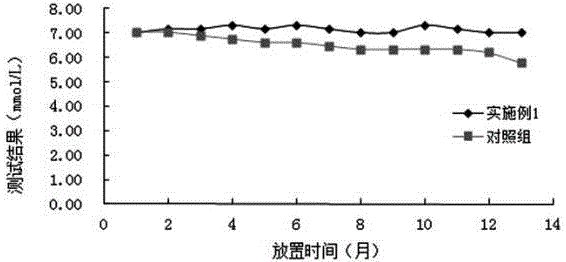
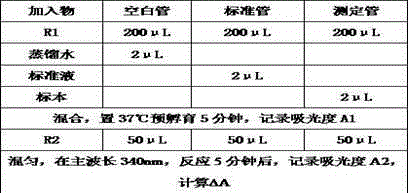
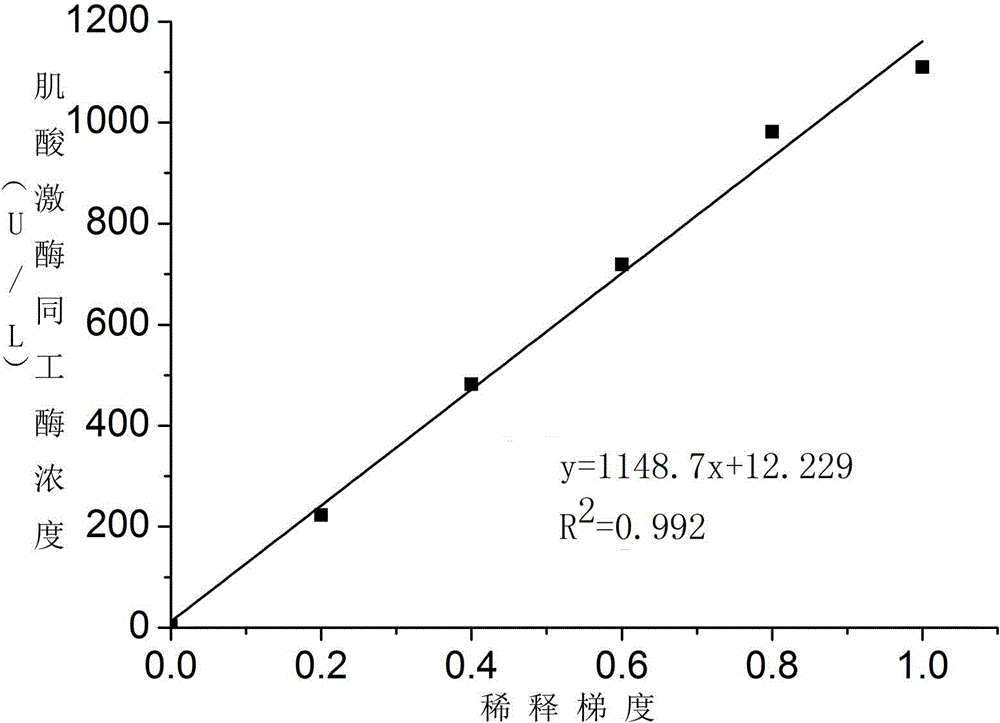
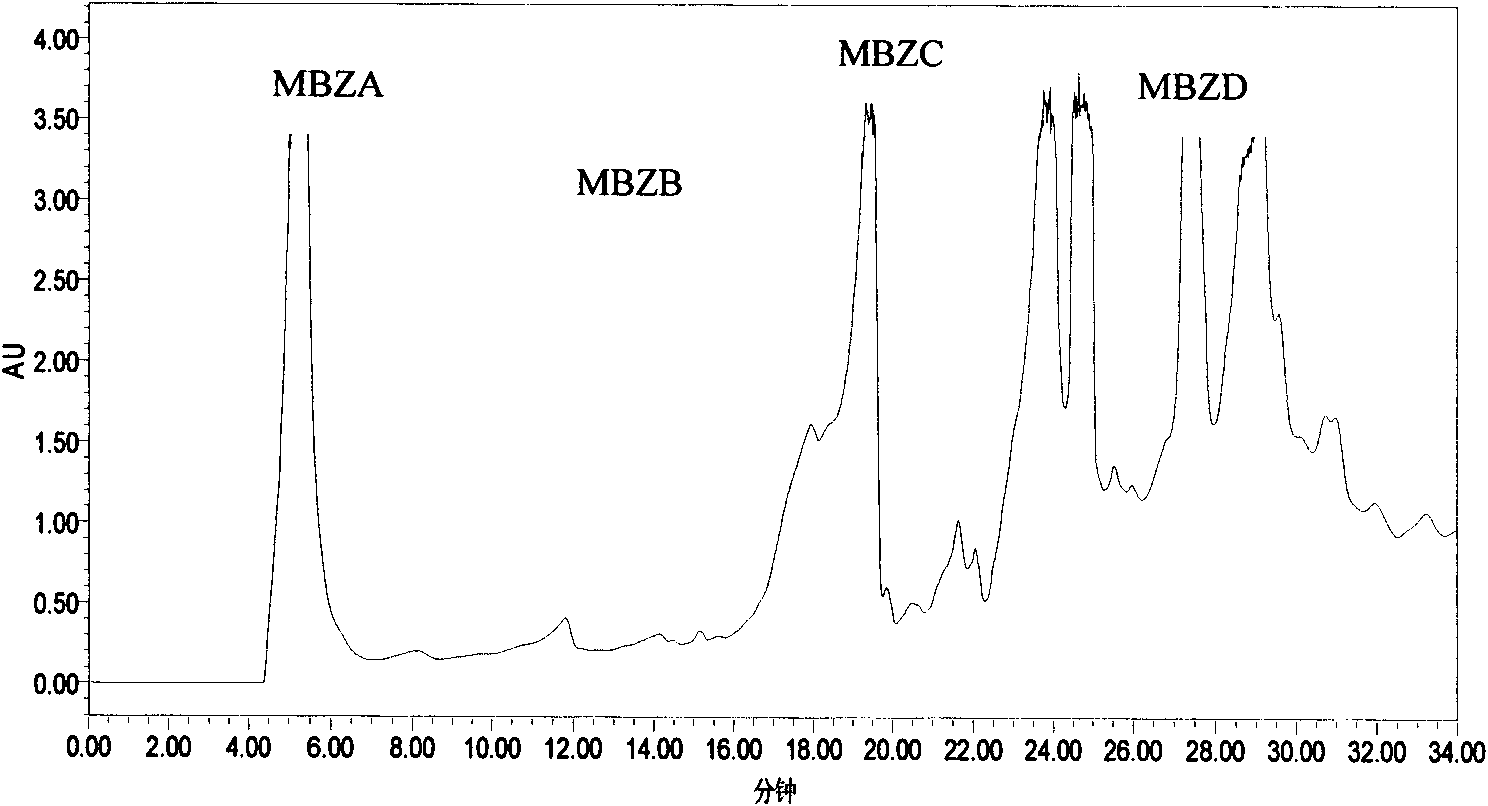
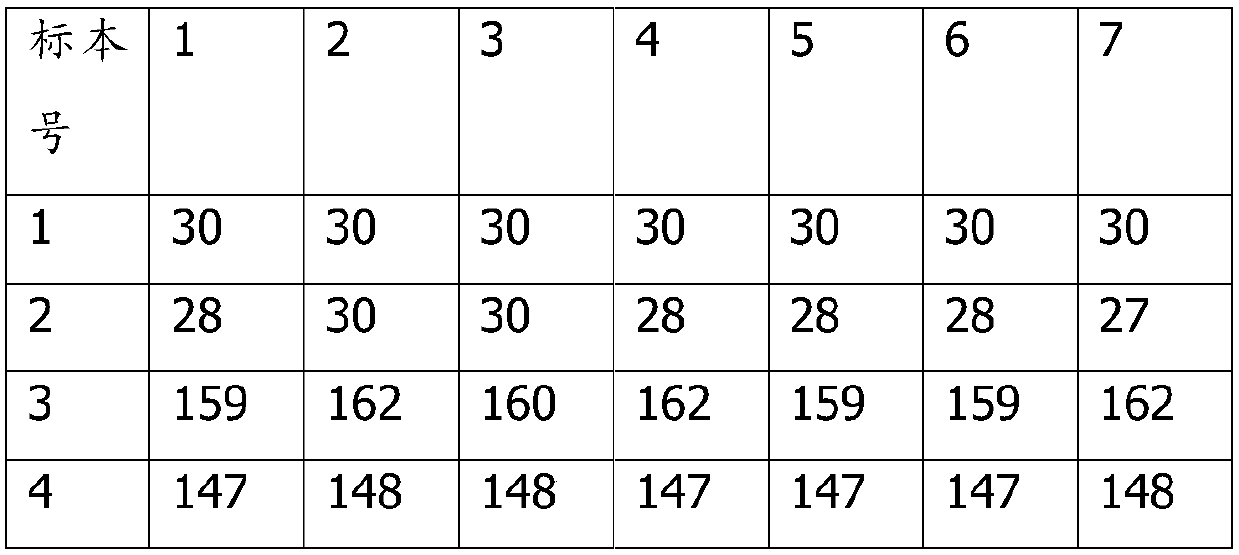
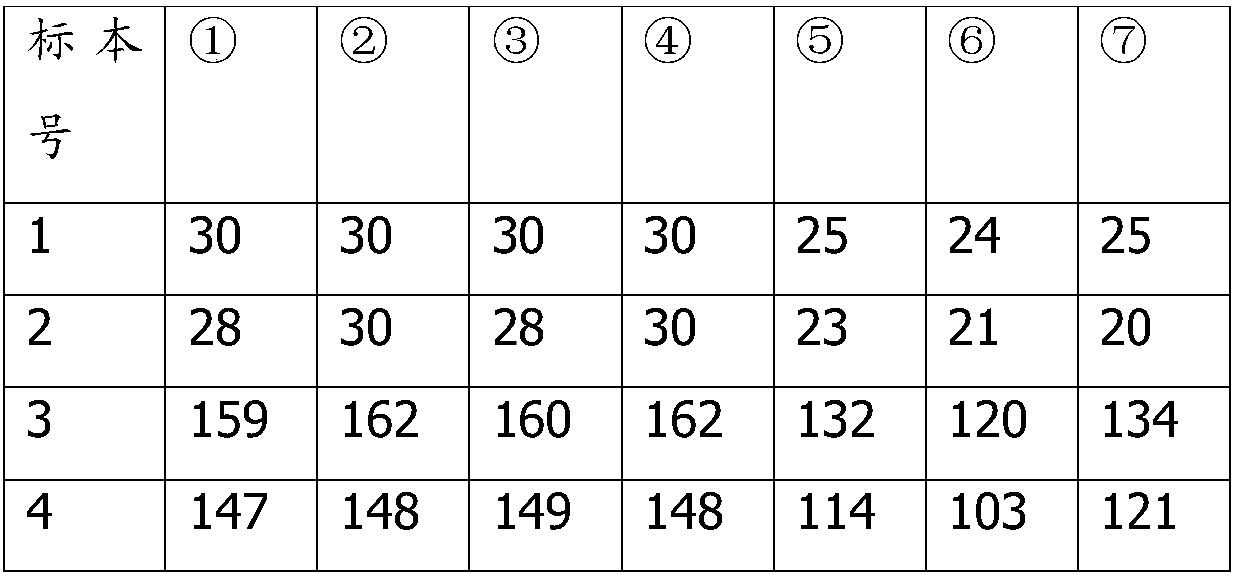
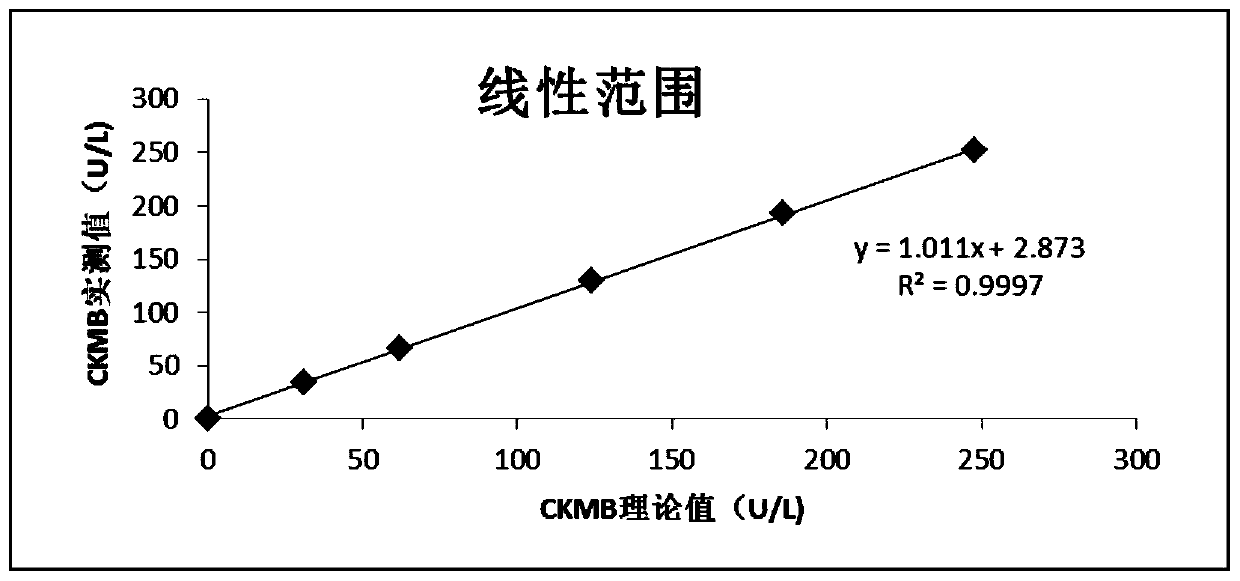
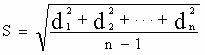Patents
Literature
138 results about "Hexokinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate of hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate.
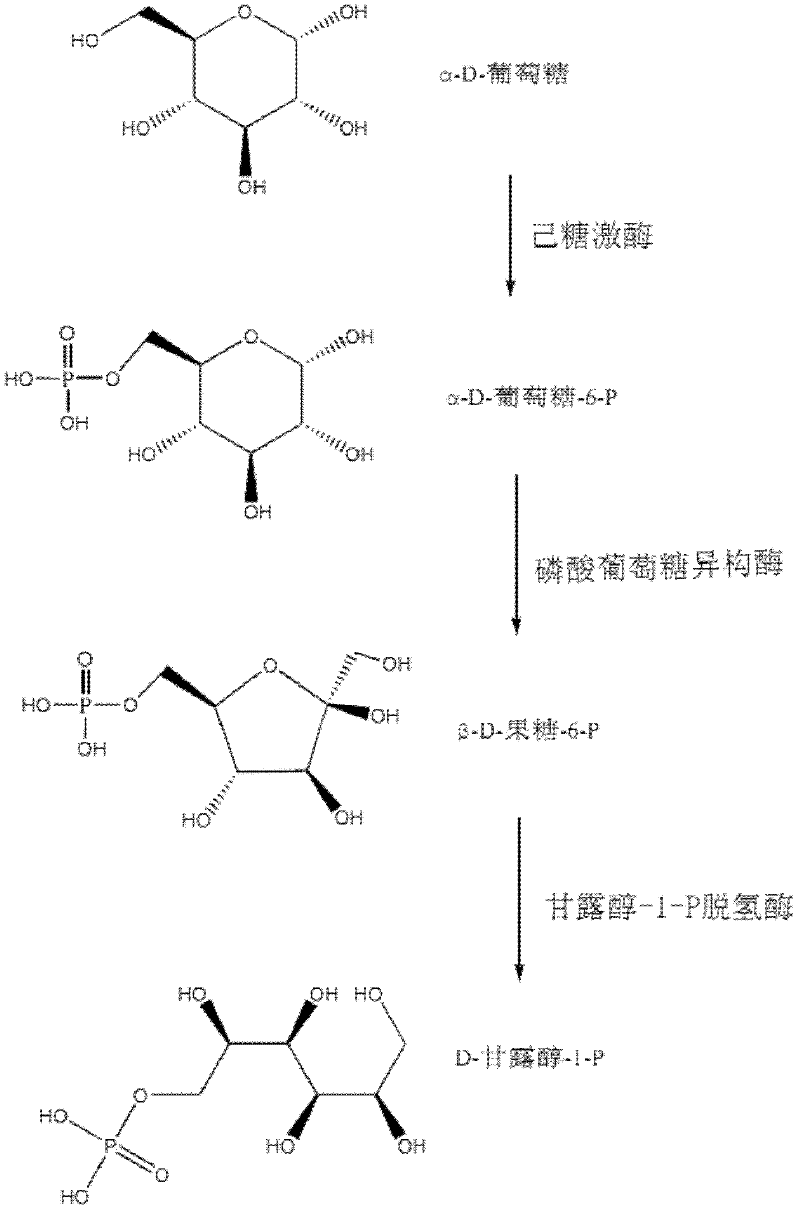
Relevant enzymes for preparing mannitol by performing anabolism on Chinese caterpillar fungus and hirsutella sinensis, gene and application thereof
The invention provides a group of relevant enzymes for preparing mannitol by performing anabolism on Chinese caterpillar fungus serving as a multifunctional production fungus and hirsutella sinensis based on glucose, a gene for encoding these enzymes and application thereof. The relevant enzymes include (1) hexokinase: manA1-A6 proteins of which the sequences are SEQ ID No.1-6, (2) phosphoglucoisomerase: manB1-B3 proteins of which the sequences are SEQ ID No.7-9, and (3) mannitol-1-P dehydrogenase: manC protein of which the sequence is SEQ ID No.10. In the invention, detailed researches are performed on the metabolic pathway of mannitol synthesized by using Chinese caterpillar fungus serving as a multifunctional production fungus, hirsutella sinensis and glucose on the aspect of principle, cloned DNA (Deoxyribose Nucleic Acid) comprising a nucleotide sequence provided by the invention can be transferred into engineering bacteria with transduction, conversion and conjugal transfer methods, and host mannitol is endowed with high expression by regulating the expression of a biosynthetic gene of the mannitol.
Owner:ZHEJIANG UNIV OF TECH +1
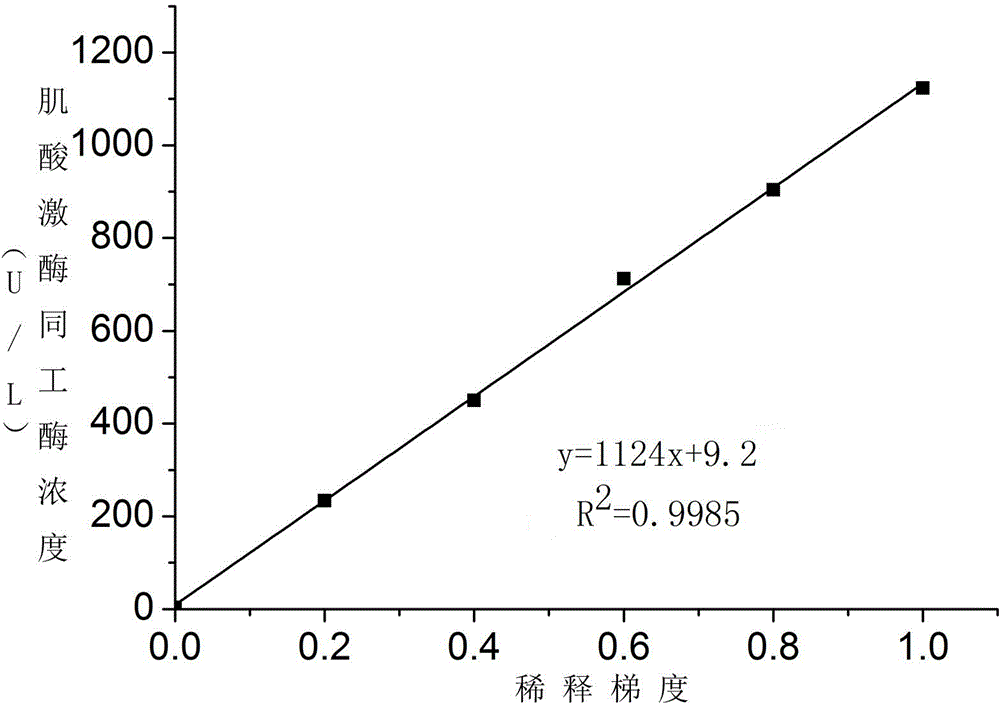
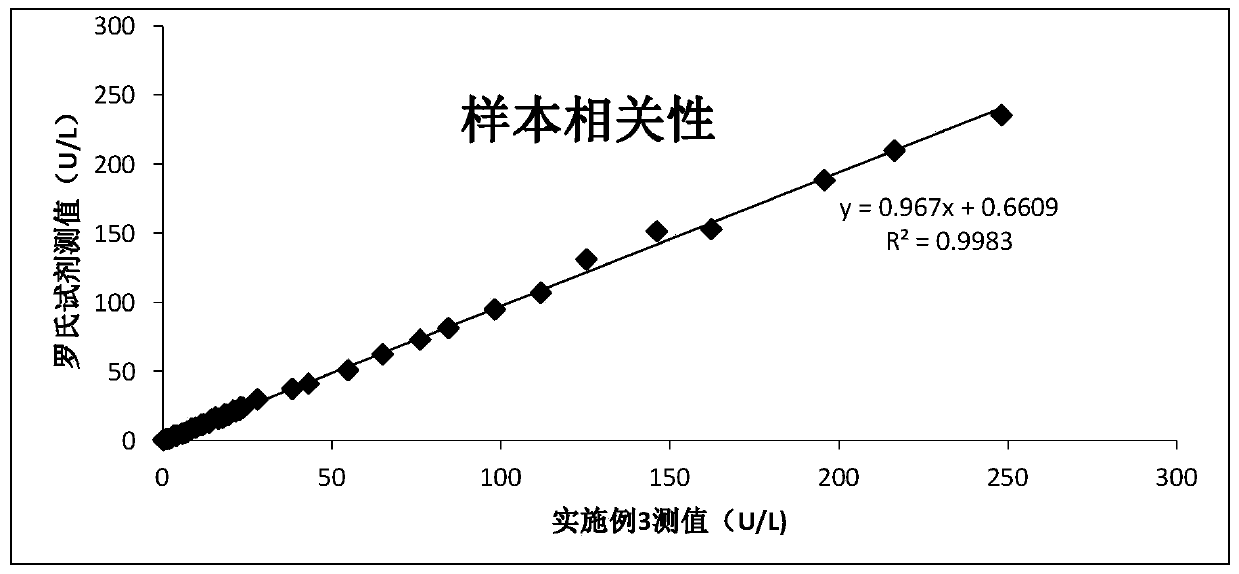
Creatine jubase MB isozyme activity detection reagent and preparation method thereof
ActiveCN102154443AHigh precisionImprove accuracyMicrobiological testing/measurementAntiendomysial antibodiesCreatine kinase
The invention discloses a creatine jubase MB isozyme activity detection reagent which comprises sulfide-oxidizing coenzyme, 6-phosphaogluconate dehydrogenase, sulfhydryl reagent, magnesium salt, glucokinase or hexoxinase, anti-CK-M antibody, phosphocreatine, adenosine diphosphate, glucose and glucose-6-phosphate dehydrogenase. The preparation method of the reagent is as follows: dissolving all components in a buffer solution with pH of 5.0 to 9.5. The creatine jubase MB isozyme activity detection reagent can enlarge detection sensitivity several times; and the determination result has high precision and degree of accuracy.
Owner:浙江东瓯诊断产品有限公司
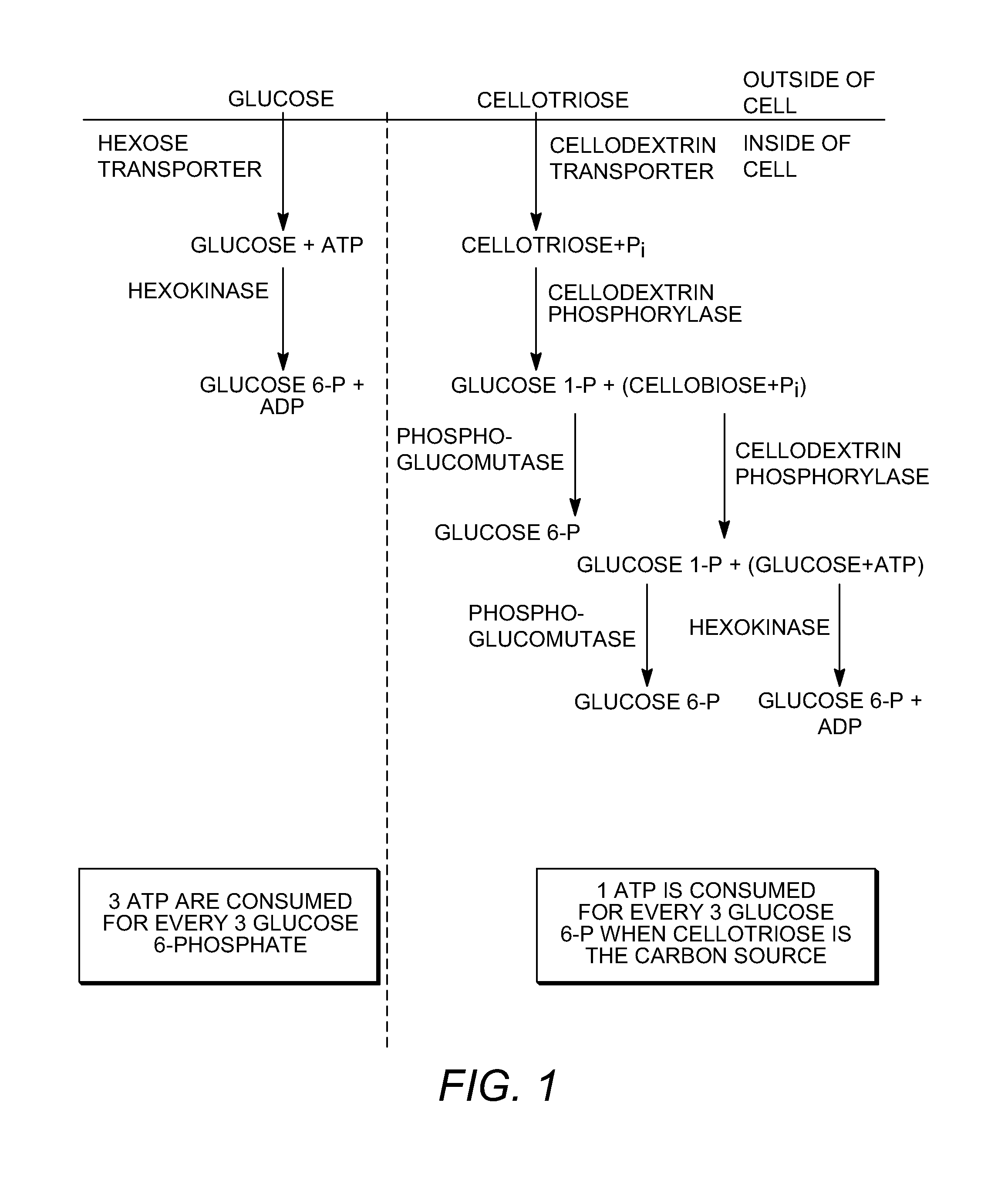
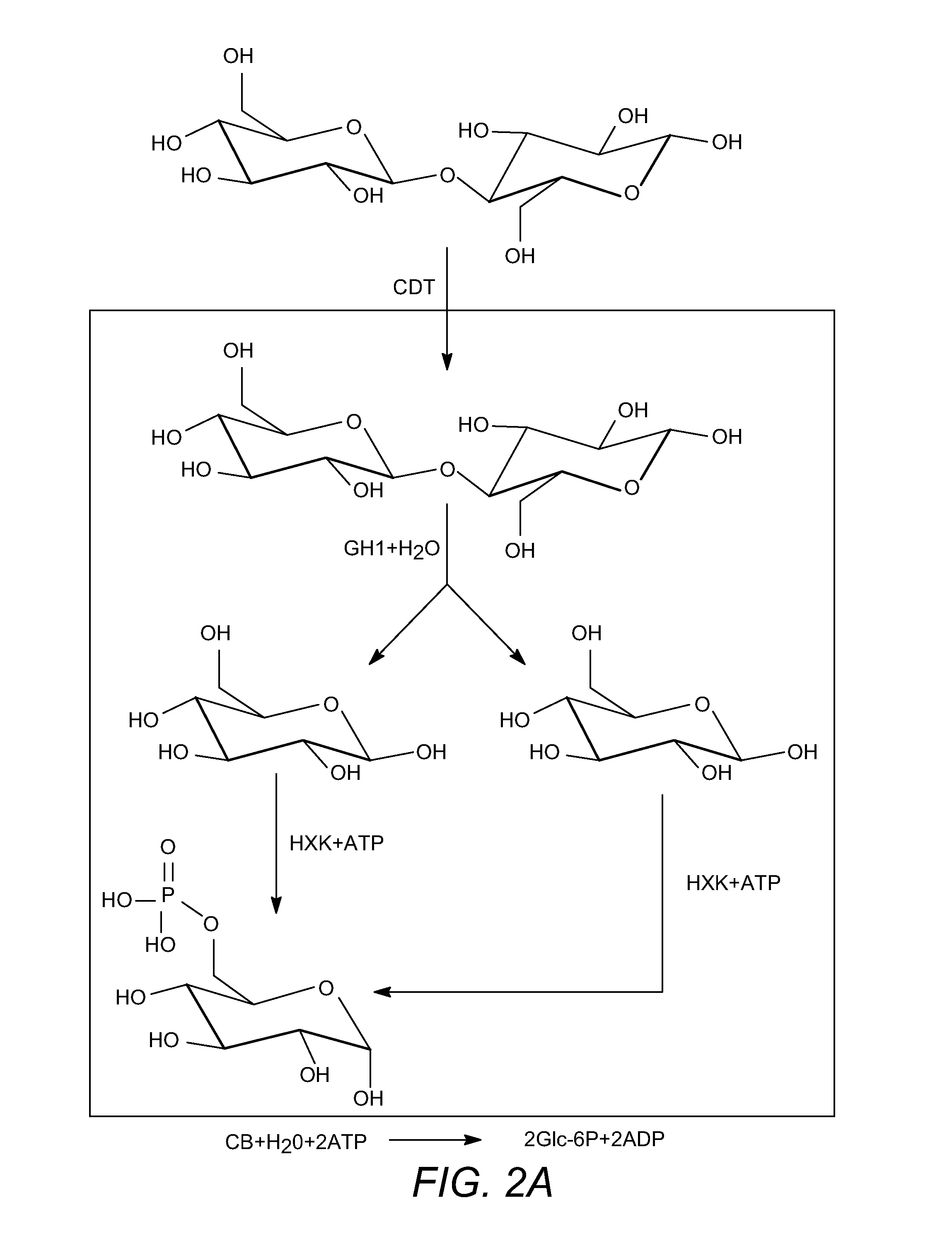
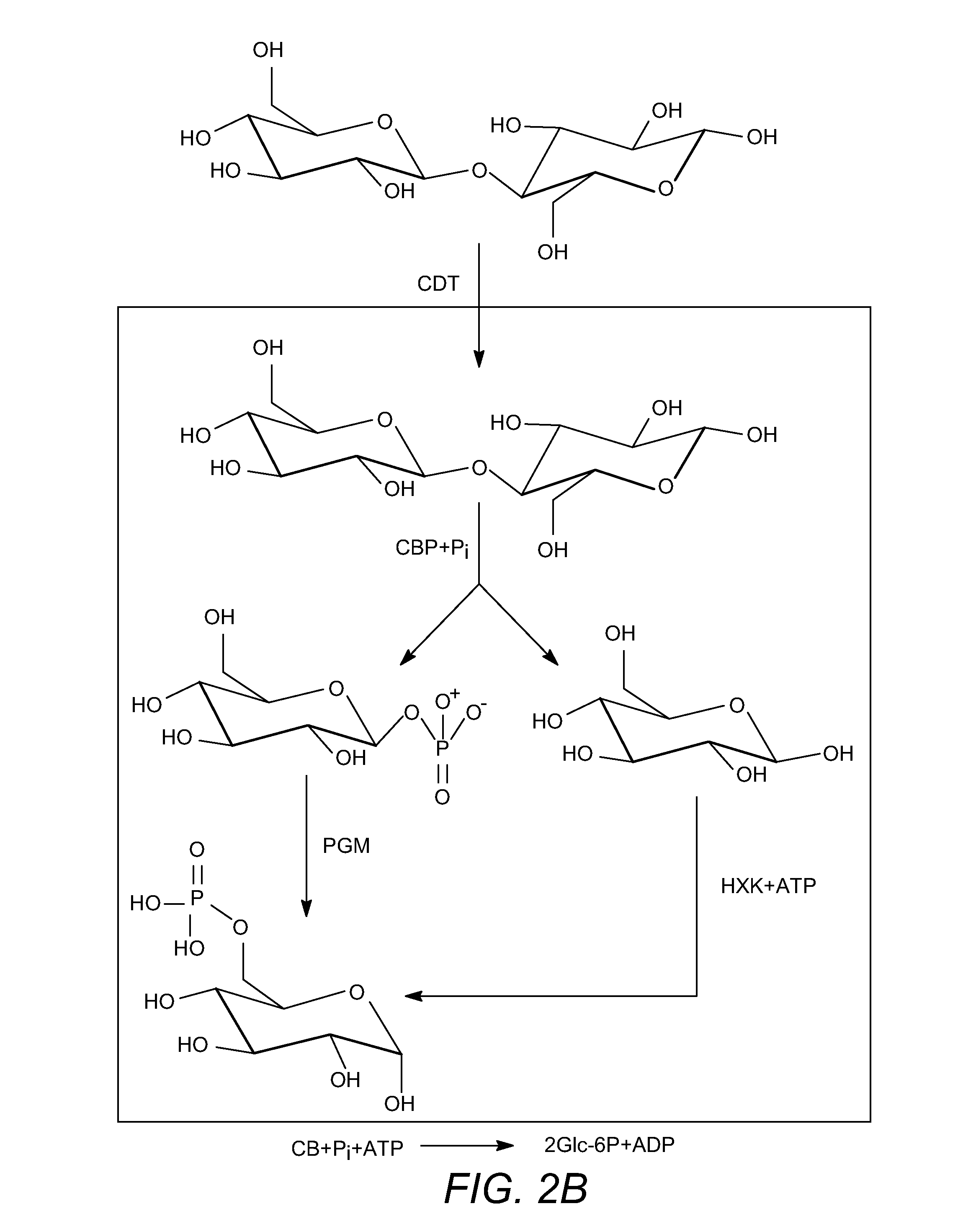
Enhanced cellodextrin metabolism
InactiveUS20140057323A1Reduce consumptionLess overall consumptionMicroorganismsBiofuelsGlucose utilizationHexokinase
The present disclosure relates to host cells containing two or more of a recombinant cellodextrin transporter, a recombinant cellodextrin phosphorylase, a recombinant β-glucosidase, a recombinant phosphoglucomutase, or a recombinant hexokinase; and to methods of using such cells for degrading cellodextrin, for producing hydrocarbons or hydrocarbon derivatives from cellodextrin, and for reducing ATP consumption during glucose utilization.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Serum AFU detection kit
ActiveCN104267178ASolve instabilityImprove protectionMicrobiological testing/measurementBiological testingPreservativeHexokinase
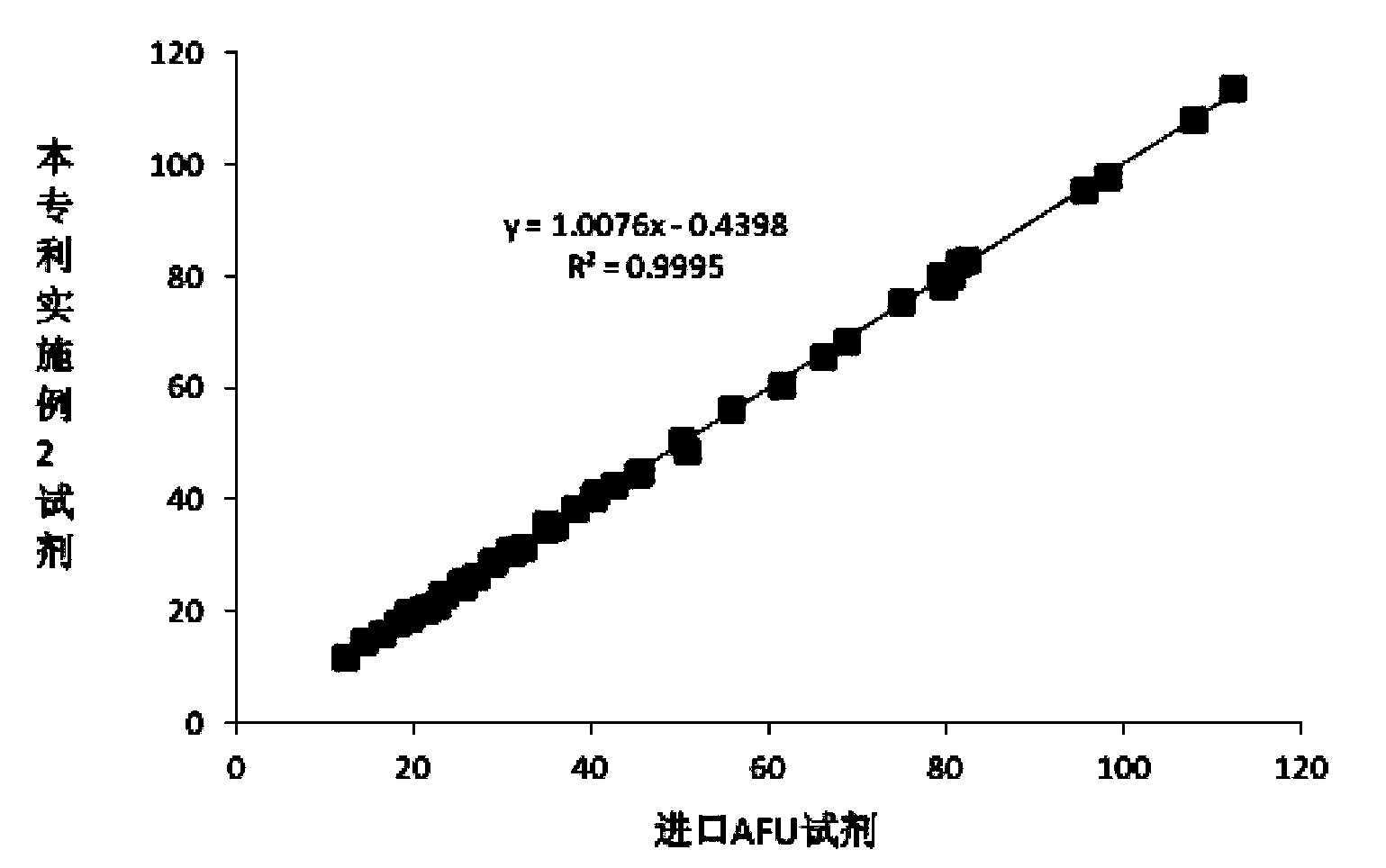
The invention relates to a serum AFU detection kit. The kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises 50-500 mmol / L of a buffer solution (pH 4.0-4.5), 1-50 g / L of a surfactant, 1-20 g / L of an anti-interference agent and 0.1-100 g / L of a preservative; the reagent 2 comprises 50-500 mmol / L of a buffer solution (pH 7.0-7.5), 2-60 g / L of glucose, 1-20 KU / L of hexokinase, 1-20 KU / L of glucose-6-phosphate dehydrogenase, 0.3-5 g / L of magnesium sulfate, 0.2-5 g / L of a substrate, 1-100 mmol / L of a stabilizer, 0.1-40 g / L of a protective agent and 0.1-100 g / L of a preservative. With the adoption of the kit, problems of the poor substrate stability, the poor anti-interference capacity and the lower sensitivity which exist universally are solved.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Aspergillus niger strain efficiently producing malic acid, construction method and application
ActiveCN111218408AIncreased purification process costsImprove efficiencyFungiTransferasesHexokinaseBatch fermentation
The invention relates to an Aspergillus niger gene engineering strain efficiently producing malic acid. The Aspergillus niger gene engineering strain is an Aspergillus niger gene engineering strain which knocks out a citric acid transporter gene cexA, and overexpresses a glucose transporter gene mstC, a hexokinase gene hxkA, a phosphofructokinase gene pfkA, and a pyruvate kinase gene pkiA which are derived from Aspergillus niger. The efficiency for producing malic acid of the Aspergillus niger gene engineering strain is significantly improved, the output on the eighth day through fed-batch fermentation in a fermentation tank reaches 195.72-210 g / L, a conversion rate of malic acid to glucose reaches 1.59-1.64 mol / mol, and a fermentation cycle is shorter by one day compared with a starting strain, namely the fermentation cycle is shortened from original 9 days to 8 days. An excellent strain for the preparation of malic acid by microbial fermentation methods is provided.
Owner:南京昊禾生物科技有限公司
Serum creatine kinase detection reagent
ActiveCN104374905AHigh activityEfficient removalMicrobiological testing/measurementBiological material analysisAcyl CoA dehydrogenaseSODIUM DODECYL BENZENE SULFONATE
The invention discloses a creatine jubase detection reagent. The reagent disclosed by the invention consists of a reagent R1 and a reagent R2 according to a volume ratio being 4 to 1, wherein the reagent R1 consists of an imidazole buffer solution, glucose, nano particles, N-acetylcysteine, ethylenediaminetetraacetic acid disodium salt, adenosine diphosphate, coenzyme I, adenine ribonucleotide, pyruvate decarboxylase, 6-phosphogluconic dehydrogenase, hexokinase and sodium dodecyl benzene sulphonate; and the reagent R2 consists of an imidazole buffer solution, phosphocreatine, a preservative and sodium dodecyl benzene sulphonate. Pyruvate decarboxylase and gamma-Fe2O3 nano particles are added into the creatine jubase detection reagent disclosed by the invention, so that interfering substances in a serum sample can be effectively eliminated, metal ions in the serum sample are chelated by matching sodium dodecyl benzene sulphonate and ethylenediaminetetraacetic acid disodium salt, the influence on reaction enzymes is alleviated, and the reagent is a stable, accurate and sensitive detection reagent with high interference resistance.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Quantitative fructose assay kit and application thereof as well as quantitative seminal plasma fructose assay method
InactiveCN102061332AEco-friendly formulaEasy to operateMicrobiological testing/measurementPreparing sample for investigationPhosphateHexokinase
The invention discloses a quantitative fructose assay kit which comprises inorganic acid deproteinized extract A, inorganic base deproteinized extract B, fructose calibration solution, a reagent 1 containing 0.001-0.1mol / L adenosine triphosphate sodium salt, a reagent 2 containing 1-100KU / L hexokinase and 1-100KU / L glucose-6-phosphate dehydrogenase, and a reagent 3 containing 0.001-0.1mol / L nicotinamide adenine dinucleotide. The seminal plasma fructose assay method comprises the following steps: respectively adding the reagent 1 and the reagent 2 to deproteinized seminal plasma and the fructose calibration solution, and mixing uniformly; reacting at the temperature of 10-40 DEG C for 5-120 minutes, then reading the absorbance respectively at the wavelength of 280-400nm; adding the reagent 3 respectively, and mixing uniformly; reacting under the same conditions and reading the absorbance; and calculating the difference between the absorbance read at the first time and the absorbance read at the second time, and comparing or calculating the absorbance of a seminal plasma specimen and the fructose calibration solution to obtain the concentration of the seminal plasma fructose. The kit and the method can be used for quantitative determination of fructose in sera, plasma, body fluid, food and solid extracting solution, the methodology is special, unique, clean and environment-friendly, manual operation and automatic batch assay can be realized, and the kit and the method are easy to popularize and apply clinically.
Owner:BRED LIFE SCI TECH
Freeze-drying concentrated glucose detection reagent microsphere and preparation method thereof
The invention discloses a freeze-drying concentrated glucose detection reagent microsphere and a preparation method thereof. The freeze-drying microsphere comprises a triethanolamine buffer solution, ATP, NADP, hexokinase, 6-glucose dehydrogenase phosphate, a preservative, trehalose, polyethylene glycol 8000, polyethylene glycol 20000, TrionX-100 and the like. The preparation method comprises the following steps: (1) preparing a solution, and performing filtration and degassing; (2) preparing droplets by using a precise quantification liquid separation system, dropwise adding the droplets into liquid nitrogen, and preparing a freezing microsphere; and (3) transferring the freezing microsphere into a freeze dryer, and performing freeze-drying on the freezing microsphere to obtain a freeze-drying detection reagent microsphere. A glucose detection reagent provided by the invention is a spherical granular freeze-drying reagent and can be pre-packaged into a detection chip. Compared with an existing liquid detection reagent, the glucose detection reagent has the advantages that the storage, transportation and the like at the stable room temperature can be realized. Compared with a freeze-drying powder reagent, the glucose detection reagent has the advantages of accuracy in quantification, convenience in packaging treatment and the like.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
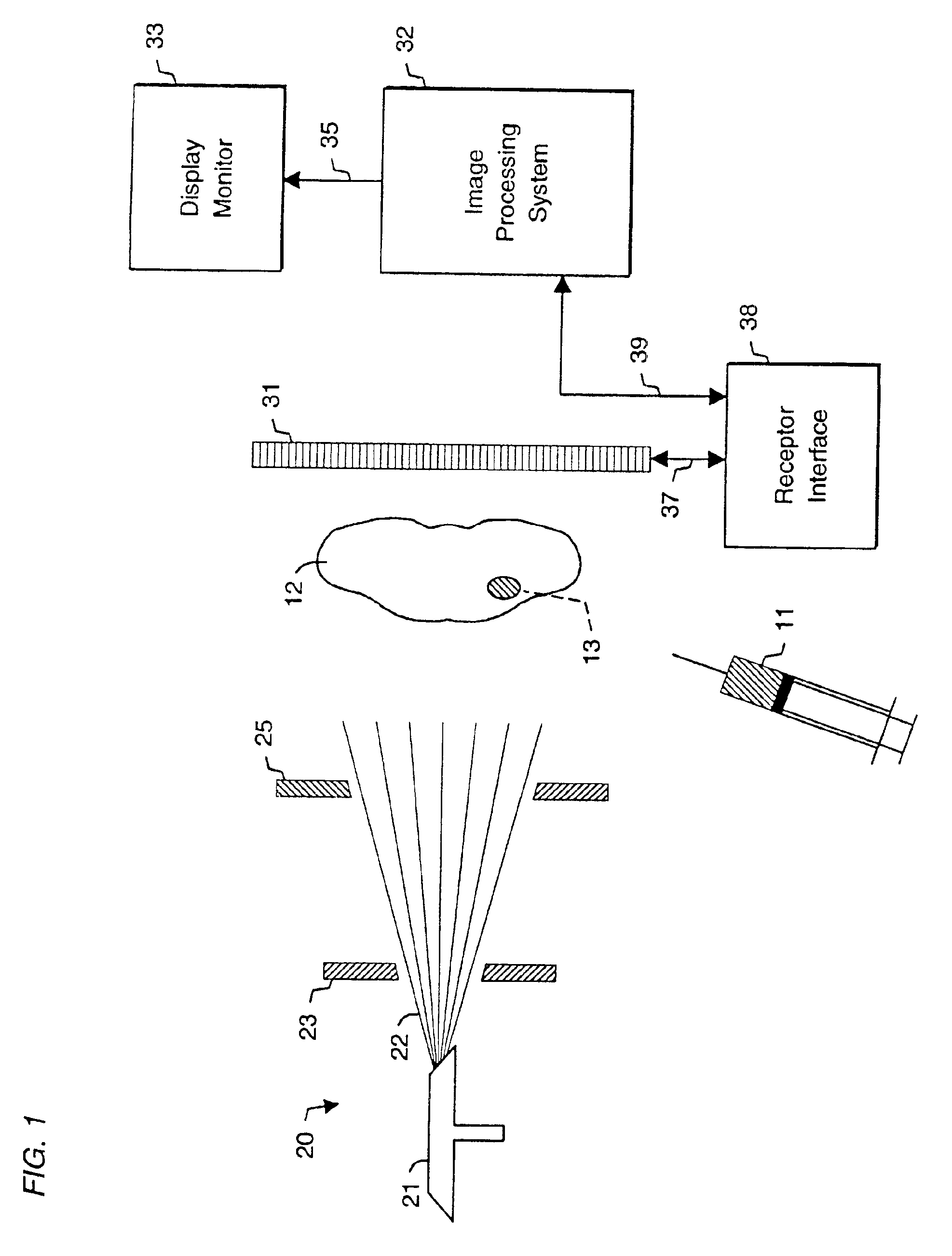
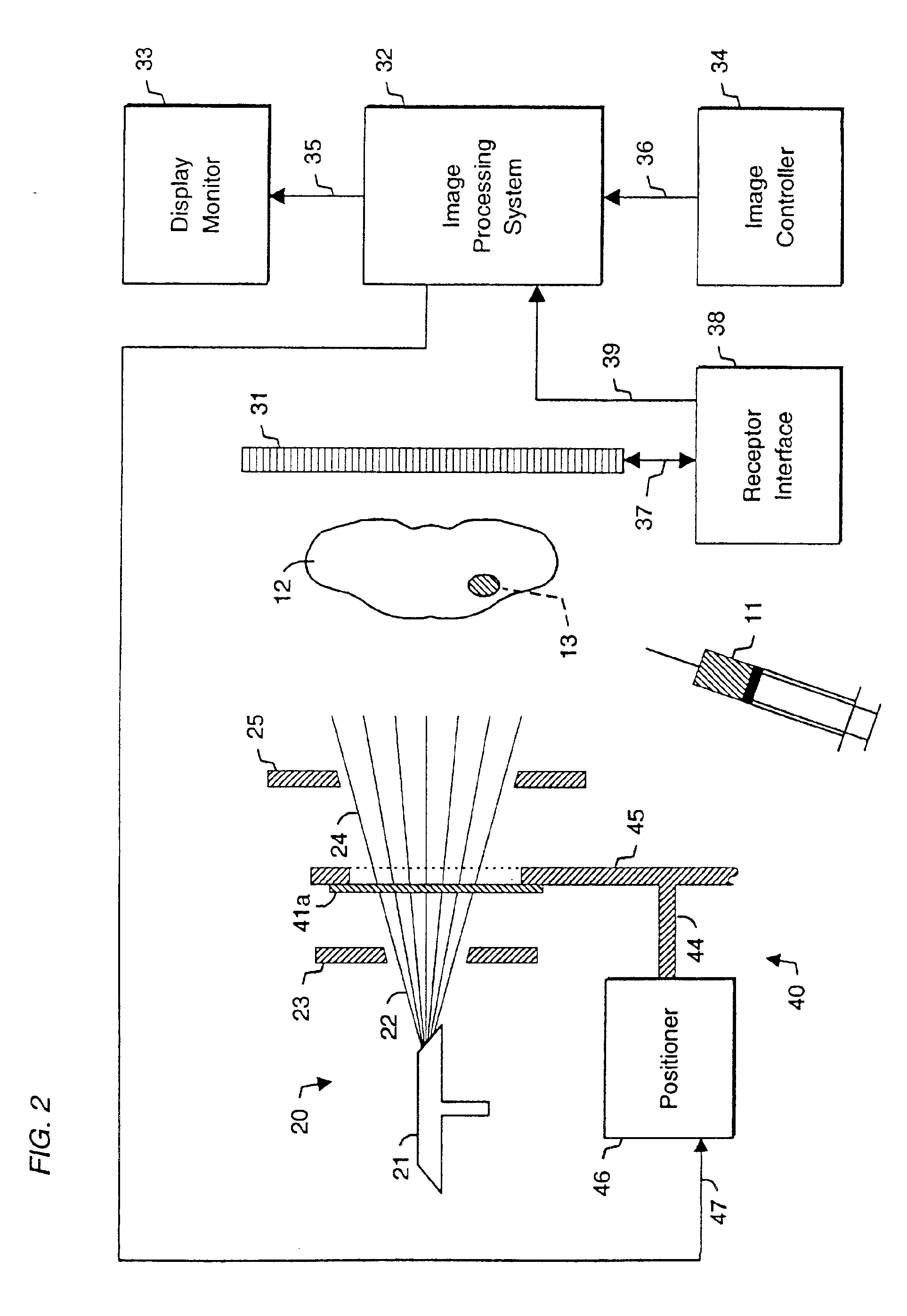
System and method for radiographic imaging of tissue
InactiveUS6923950B2High anatomical detailHigh spatial resolutionIn-vivo radioactive preparationsSugar derivativesAnatomical structuresX-ray
System and method for radiographic imaging of tissue using a non-radioactive, radio-opaque imaging agent that accumulates intracellularly in tissue in proportion to its functional, or physiological, activity. In one embodiment, the imaging agent is a cell-membrane permeable, radio-opaque, high affinity ligand for the intracellular enzyme hexokinase. The imaging agent is administered to a patient, and after an accumulation interval, radiographic images are acquired. The imaging agent preferentially accumulates in malignant tissue and increases its radio-opacity because of its elevated glucose metabolic rate relative to benign and normal tissue. The tissue being examined is transilluminated by X-ray beams with preselected different mean energy spectra, and a separate radiographic image is acquired during transillumination by each beam. An image processing system performs a weighted combination of the acquired images to produce a single displayed image. The image processing procedure isolates the radiographic density contributed solely by differential accumulation of the imaging agent in malignant, benign, and normal tissue. The system and method thus provides a functional image displayed with the anatomical detail and spatial resolution of a radiographic image. The viewer may interactively control the relative proportion of radiographic density contributed by imaging agent, soft tissue, and bone to the displayed image, allowing the display of functional and anatomical information in complete registration, and facilitating localization of malignant tissue in relation to nearby anatomical structures. In other embodiments, the system and method may be used to detect enzymes, nucleic acids, coenzymes, fatty acids, and other cellular targets in diagnostic imaging applications.
Owner:VERITAS PHARM INC
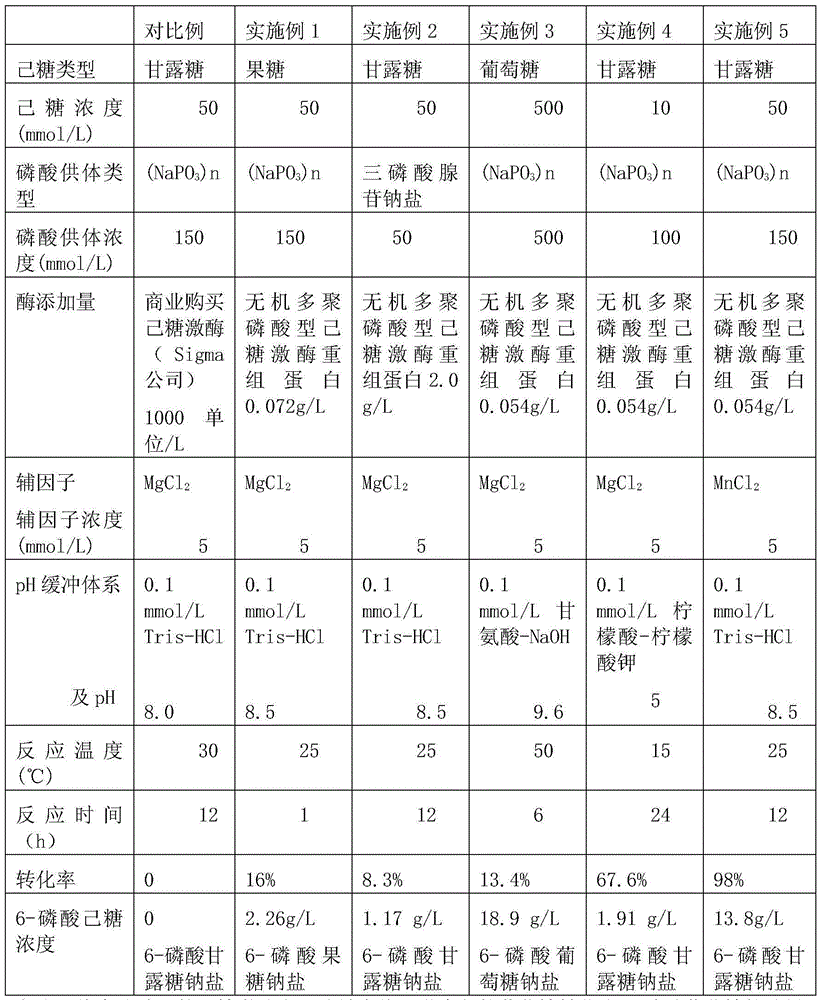
Method for synthesizing 6-hexose phosphate by employing enzymic method
InactiveCN104673855AHigh catalytic efficiencyMild reaction conditionsTransferasesFermentationPhosphatePolyphosphate
The invention relates to a method for synthesizing 6-hexose phosphate by employing an enzymic method. The method comprises the following steps: with hexose as a substrate, polyphosphates and / or triphosadenine salt as phosphate donors, putting a reactant into a pH buffer system; adding inorganic polyphosphoric acid hexokinase and cofactors to the buffer system; and synthesizing the 6-hexose phosphate under the catalysis action of the inorganic polyphosphoric acid hexokinase. Compared with a chemical method, the method for synthesizing the 6-hexose phosphate by employing the enzymic method is green and safe; compared with other methods for synthesizing the 6-hexose phosphate by other enzymic methods, the polyphosphates and / or triphosadenine salt can be used as phosphate donors by the inorganic polyphosphoric acid hexokinase disclosed by the invention. The triphosadenine salt with high cost is replaced with polyphosphates, so that the problem of high production cost is solved.
Owner:BEIJING UNIV OF CHEM TECH
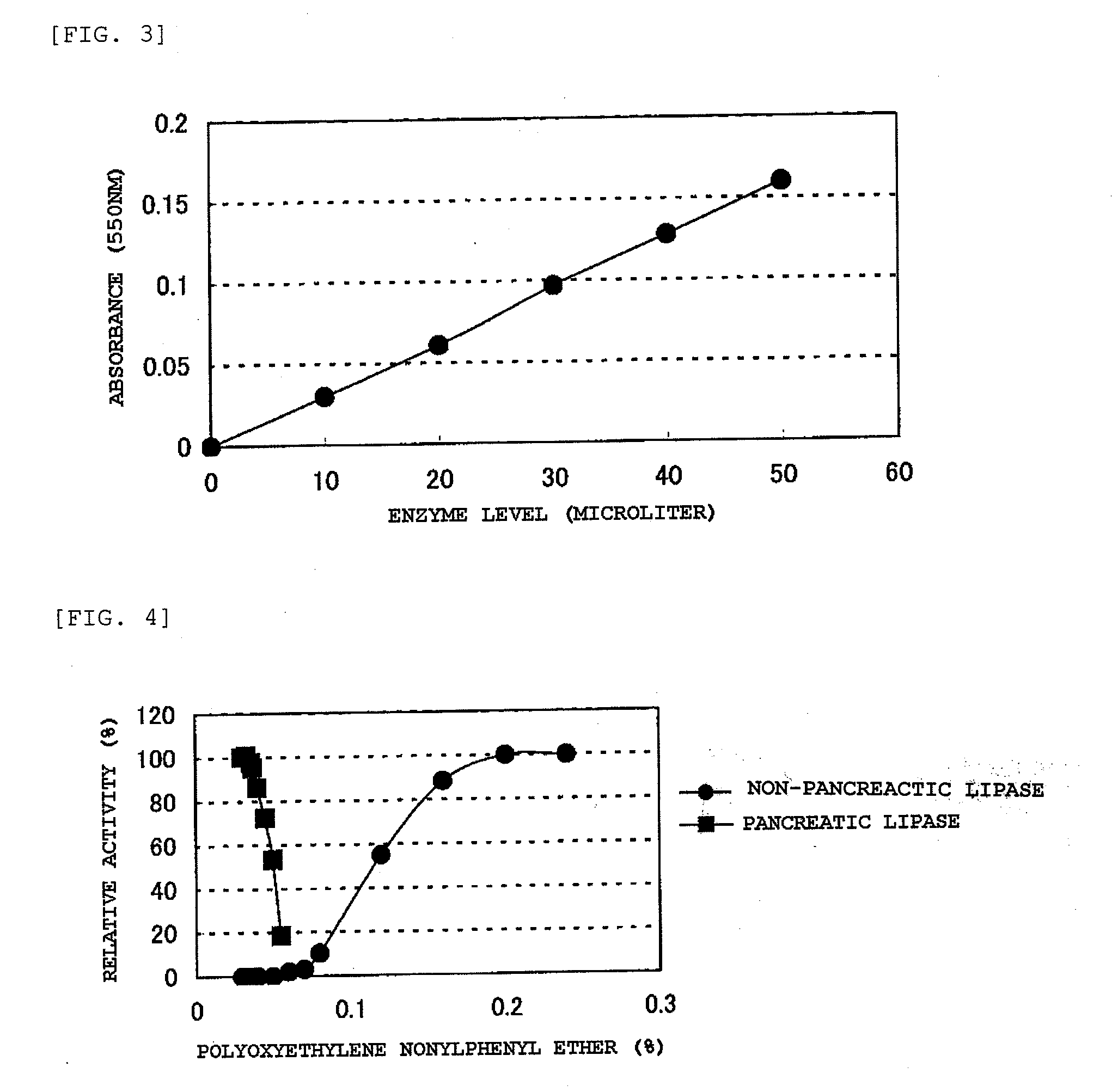
Composition for Lipase Activity Determination and Method of Determing Activity
InactiveUS20080038765A1Easy to useGood reproducibilityOrganic chemistryMicrobiological testing/measurementMonoglycerideClinical exam
[PROBLEMS] To provide lipase activity determination reagents which function by an enzymatic method. The reagents are easy to use in an ordinary clinical examination, have excellent handleability, and are excellent in accuracy and reproducibility. [MEANS FOR SOLVING PROBLEMS] Lipase activity is determined with any of reagents which comprise a low-concentration buffer and a diglyceride dissolved therein. The diglyceride is used as a substrate for lipase, whereby the liquid reagents can have long-term storage stability. One of the reagents converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, pyruvate kinase, lactate dehydrogenase, reduced NAD, ATP, and phosphoenol pyruvate. Another reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glucose, ADP-dependent hexokinase, glucose-6-phosphate dehydrogenase, oxidized NAD or oxidized NADP, and ATP. A further reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glycerol-3-phosphate oxidase, peroxidase, and a dye which colors in the presence of hydrogen peroxide.
Owner:ASAHI KASEI PHARMA
Glucose detection reagent being strong in stability and low in cost and adopting hexokinase
ActiveCN106868096AEasy to storeImprove protectionMicrobiological testing/measurementPolyethylene glycolHexokinase
The invention relates to the technical field of detection for GLU in the blood by adopting hexokinase, and in particular relates to a detection reagent for GLU in the blood, which is strong in stability and low in cost and adopts liquid hexokinase. The detection reagent adopts liquid double reagents, wherein the reagent I mainly contains a buffer solution, Mg<2+>, an ion balancing agent, a preservative, NAD (oxidized coenzyme I), a protective agent and a surfactant, and the reagent II contains a buffer solution, a protective agent, a surfactant, preservative, hexokinase (HK), glucose-6-phosphate dehydrogenase (G6P-DH), ATP.NA2, and the like. In order to guarantee the stability of enzymes in the reagent, various protective agents including polyethylene glycol-20000, tween-80, FAD and the like are added in the reagent in a pertinence manner, in order to reduce the cost, especially the use of enzymes, various surfactants are added in the reagent, so that the emulsifying for enzymes is enhanced, the service efficiency for enzymes is improved, thus the cost of the reagent is greatly reduced, and the detection reagent is particularly suitable for being used and popularized in clinic.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Serum glucose detection reagent
ActiveCN105063174AEnsure stabilityImprove stability and anti-interference abilityMicrobiological testing/measurementSucroseAdenosine
The invention discloses a serum glucose detection reagent which is composed of a reagent R1 and a reagent R2, wherein the reagent R1 is composed of a buffer solution, Mg<2+>, adenosine triphosphate (ATP), NAD+, a fluorocarbon surfactant, BSA (bovine serum albumin), sucrose, trehalose, mannitol and a preservative; and the reagent R2 is composed of a buffer solution, Mg<2+>, BSA, sucrose, trehalose, mannitol, glucose-6-phosphate dehydrogenase, hexokinase, a fluorocarbon surfactant and a preservative. The reagent has the advantages of high accuracy, high stability and low price, is convenient to use and can completely satisfy the clinical demands.
Owner:郁东
Enzymatically grafted phosphorus-containing chitosan oligosaccharide based inflaming retarding finishing method of protein fiber product
ActiveCN106978720AHigh catalytic efficiencyImprove reaction efficiencyHeat resistant fibresBiochemical treatment with enzymes/microorganismsHexokinasePre treatment
The invention discloses an enzymatically grafted phosphorus-containing chitosan oligosaccharide based inflaming retarding finishing method of a protein fiber product, and belongs to the technical field of textile biology. The inflaming retarding finishing method aims at solving defects that the fiber damage and the like are easily caused when a conventional chemical method is used for carrying out the inflaming retarding finishing on the protein fiber product. According to the inflaming retarding method, the pretreatment of the protein fiber product is first carried out; chitosan oligosaccharide is catalyzed to be grafted on the surface of a protein fiber through tyrosinase; the chitosan oligosaccharide is catalyzed to be phosphorylated by combining with hexokinase; the inflaming retarding effect is given to the protein fiber product. The inflaming retarding finishing method comprises the following specific steps of (1), carrying out the pretreatment on the protein fiber product; (2), catalyzing the protein fiber to graft the chitosan oligosaccharide by the tyrosinase; (3), catalyzing the chitosan oligosaccharide to be phosphorylated by the hexokinase; (4), carrying out water scrubbing and oven-drying post treatment. The protein fiber product treated by the inflaming retarding method is ameliorated in inflaming retarding performance and improved in mechanical performance; in comparison with the inflaming retarding finishing of the conventional chemical method, the inflaming retarding finishing, by which the enzymatically grafted phosphorus-containing chitosan oligosaccharide is adopted, of the protein fiber product is low in energy consumption.
Owner:JIANGNAN UNIV
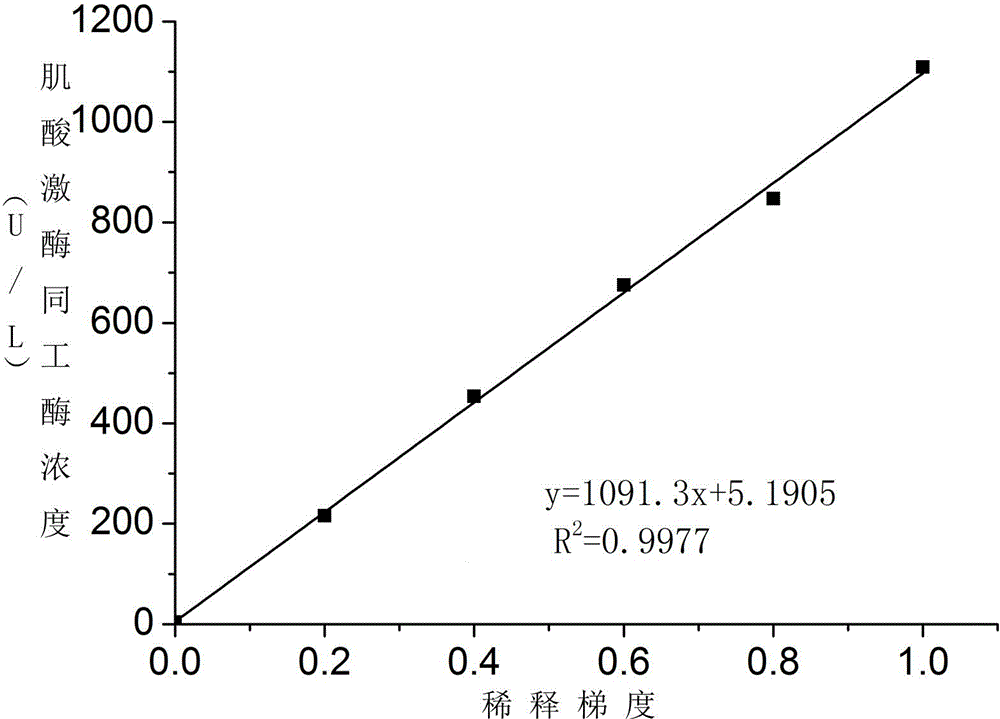
Creatine kinase isoenzyme detection reagent
ActiveCN104374925AHigh activityEfficient removalBiological testingSodium acetateAntiendomysial antibodies
The invention discloses a creatine kinase isoenzyme detection reagent. The reagent consists of a reagent R1 and a reagent R2 according to the volume ratio of 4 to 1, wherein the reagent R1 consists of an imidazole buffer liquid, glucose, nano particles, N-acetylcysteine, sodium ethylene diamine tetracetate, adenosine diphosphate, cozymase I, ribonucleotide, pyruvate decarboxylase, glucose 6-phosphate dehydrogenase, hexokinase, a goat anti-human CK-M polyclonal antibody and trehalose; the reagent R2 consists of an imidazole buffer liquid, phosphocreatine, sodium dodecyl benzene sulfonate and a preservative. The reagent disclosed by the invention is a creatine kinase isoenzyme detection reagent which is stable, accurate, and high in anti-interference, and has very high clinical application value.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
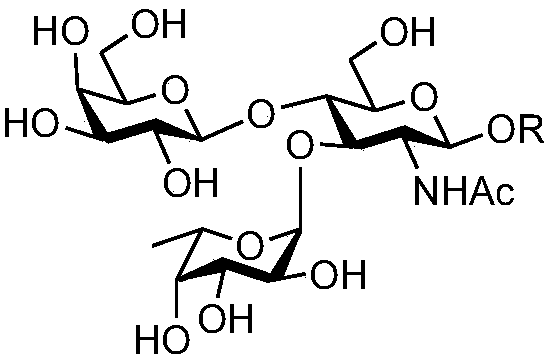
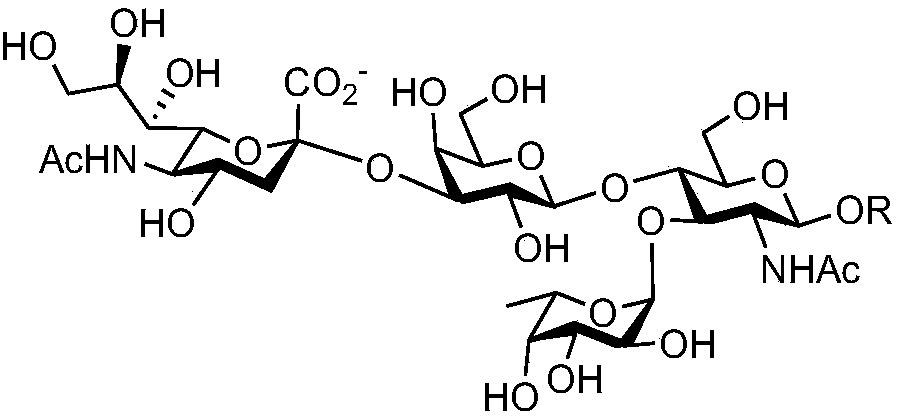
Synthesis method of oligosaccharide of Lewis x unimer, dimer and saliva acidifying derivatives thereof
InactiveCN108130349AEfficient synthesisSynthesis fastFermentationSialic acid aldolaseSynthesis methods
The invention discloses a synthesis method of oligosaccharides of a Lewis x unimer, a dimer and saliva acidifying derivatives thereof. The method comprises the steps that core framework compounds (V)to (IX) are built by enzymic method modular assembly 1 and enzymic method modular assembly 2; a Lex unimer and a dimer (I) to (II) are assembled by enzymic method modular assembly 3; finally, an sLexunimer and A dimer (III) to (IV) are built by enzymic method modular assembly 4. High region selectivity and high efficiency of the enzymic method synthesis are combined; the target molecule synthesisis realized at higher yield. Glycosyltransferase, riboside generation enzyme and hexokinase are from prokaryotic organisms; the advantages of high protein expression, wide substrate adaptability, high catalysis efficiency and the like are realized; the synthesis method can be used for large-scale preparation.
Owner:SHANDONG UNIV
Compositions for lipase activity determination and method of determining activity
InactiveCN101061234AEasy to useExcellent long-term storage stabilityMicrobiological testing/measurementLactate dehydrogenaseMonoglyceride
To provide lipase activity determination reagents which function by the enzymatic method. The reagents are easy to use in an ordinary clinical examination, have excellent handleability, and are excellent in accuracy and reproducibility. Lipase activity is determined with any of reagents which comprise a low-concentration buffer and a diglyceride dissolved therein. The diglyceride is used as a substrate for lipase, whereby the liquid reagents can have long-term storage stability. One of the reagents converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, pyruvate kinase, lactate dehydrogenase, reduced NAD, ATP, and phosphoenol pyruvate. Another reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glucose, ADP-dependent hexokinase, glucose-6-phosphate dehydrogenase, oxidized NAD or oxidized NADP, and ATP. A further reagent converts a monoglyceride yielded by a lipase reaction into glycerol with the aid of monoglyceride lipase, and further contains glycerol kinase, glycero-3-phosphate oxidase, peroxidase, and a dye which colors in the presence of hydrogen peroxide.
Owner:ASAHI KASEI PHARMA
Method for modifying and promoting dyeing property of protein fiber product through two-step process
ActiveCN108894012AEnvironmentally friendlyImprove reaction efficiencyBiochemical treatment with enzymes/microorganismsDyeing processPhosphorylationHexokinase
The invention discloses a method for modifying and promoting a dyeing property of a protein fiber product through a two-step process. Reduced hexaose is grafted onto a protein fiber through schiff base reaction, and then hexokinase is utilized to catalyze hexaose phosphorylation, so that the attachment of cationic dyes to the protein fiber dyeing can be boosted and the dyeing property of the protein fiber products is promoted. The method comprises the following steps: (1) grafting hexaose to the protein fiber: performing one-bath treatment on the protein fiber product and hexaose and graftinghexaose to the protein fiber through schiff base reaction; (2) catalyzing hexaose phosphorylation with hexokinase: washing the protein fiber product treated in the step (1) with water, and then treating hexokinase and triphosadenine. Compared with the cationic dyeing realized in the manner of adopting a chemical method for introducing electronegative groups onto the protein fiber, the method disclosed by the invention has the advantages that the hexaose is environment-friendly, the enzymic catalytic reaction efficiency is high and the dyeing property of the protein fiber product is obviously improved.
Owner:JIANGNAN UNIV
Genetic engineering bacteria producing beta- carotene and construction method of genetic engineering bacteria
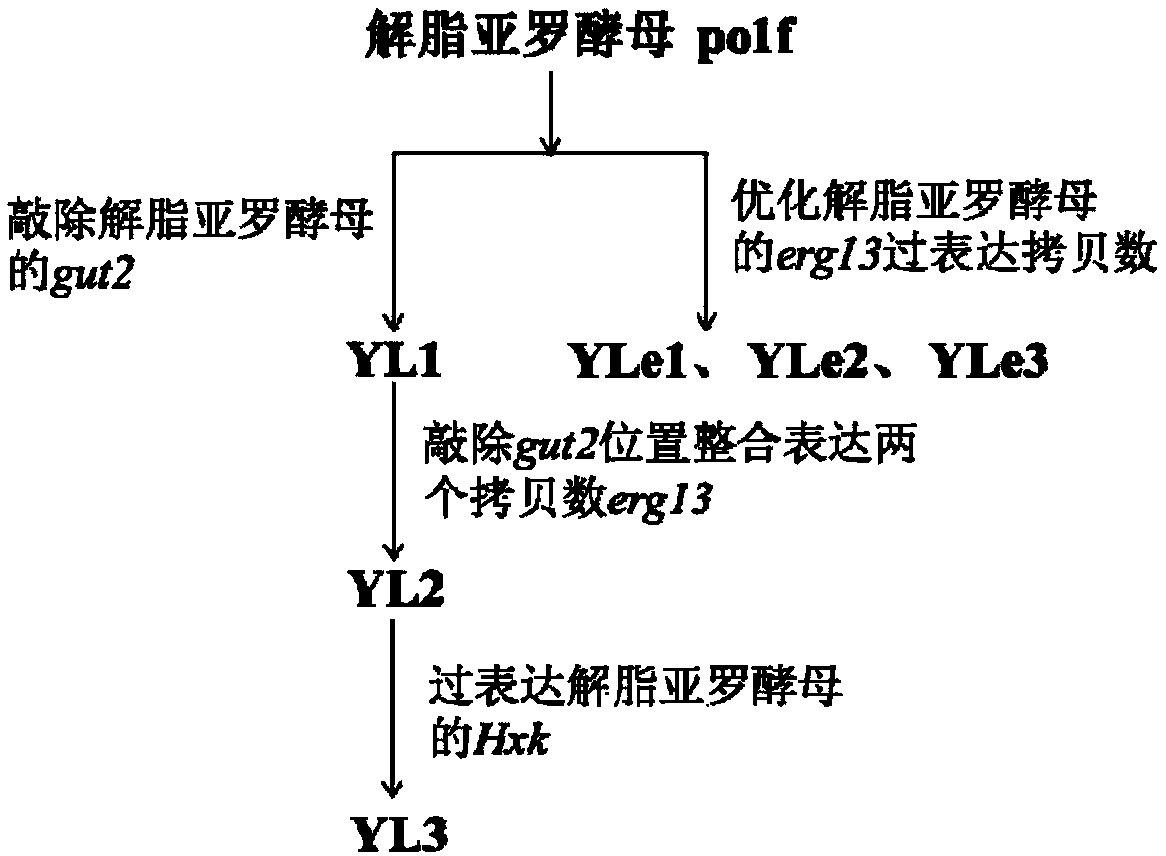
ActiveCN109609579AIncrease storage capacityIncrease productionFungiMicroorganism based processesBeta-CaroteneHexokinase
The invention discloses genetic engineering bacteria producing beta- carotene and a construction method of the genetic engineering bacteria. The construction method comprises the following steps of firstly knocking out alpha-glycerophosphate dehydrogenase gut2 genes in a glycolysis way in a beta-carotene synthetizing pathway in yarrowia lipolytica, then performing free expression on beta-hydroxyl-beta-methylglutaric acid erg13 genes having two endogenous copy numbers of the yarrowia lipolytica, and then performing integrant expression on the erg13 genes having two endogenous copy numbers of the yarrowia lipolytica at the position where the gut2 genes are knocked out; and performing free expression on endogenous hexokinase Hxk genes of the yarrowia lipolytica, and screening positive transformants to obtain the engineering bacterial strains producing beta-carotene yarrowia lipolytica. The bacterial strains are subjected to fermentation, culturing, extraction and separation, the beta-carotene is purified, and the content of the beta-carotene can reach 26.6mg / g cell dry weight.
Owner:SHAANXI NORMAL UNIV
Method for preparing diagnostic hexokinase
PendingCN106479992AThe preparation method is feasibleHigh purityFungiTransferasesIntellectual propertyHexokinase
The invention belongs to production of enzymic preparations and particularly relates to a method for preparing diagnostic hexokinase. The method includes the process steps of purifying and rejuvenating a strain, preparing fermentation liquor containing hexokinase by fermentation and purifying the fermented crude hexokinase enzyme. The method for preparing the diagnostic hexokinase has the advantages that the problems that the diagnostic hexokinase with independent intellectual property is not available domestically, imported enzymic preparations are high in price and inconvenient to use and the like currently are solved, the method is reliable in strain source, feasible, suitable for industrialized production and capable of filling the blank that the diagnostic hexokinase with the independent intellectual property is not available domestically, and major performance indexes of the diagnostic hexokinase prepared by the method are superior to those of the imported products.
Owner:河北省微生物研究所有限公司
Method for synthesizing of 2'-fucosyllactose
InactiveCN110172486AReduce manufacturing costIncrease productivityMicroorganism based processesFermentationBiotechnologyStaphylococcus lactis
The invention belongs to the field of genetic engineering and relates to a method for producing 2'-fucosyllactose, in particular to a method for synthesizing 2'-fucosyllactose by co-catalysis of a genetically engineered strain and enzyme. The method of the invention comprises the steps of using a recombinant strain of lactococcus lactis which efficiently expresses hexokinase, phosphomannomutase and mannose-1-phosphate guanyl transferase as a production strain, and using mannose as a substrate to synthesize GDP-mannose, and then using GDP-mannose 4,6-dehydratase, GDP-4-keto-6-deoxymannose 3,5-mutarotase / 4-reductase, alpha1,2-fucosyltransferase to catalyze GDP-mannose in vitro to synthesize the 2'-fucosyllactose, thus providing a new method for the industrial production of the 2'-fucosyllactose.
Owner:TIANJIN UNIV OF SCI & TECH
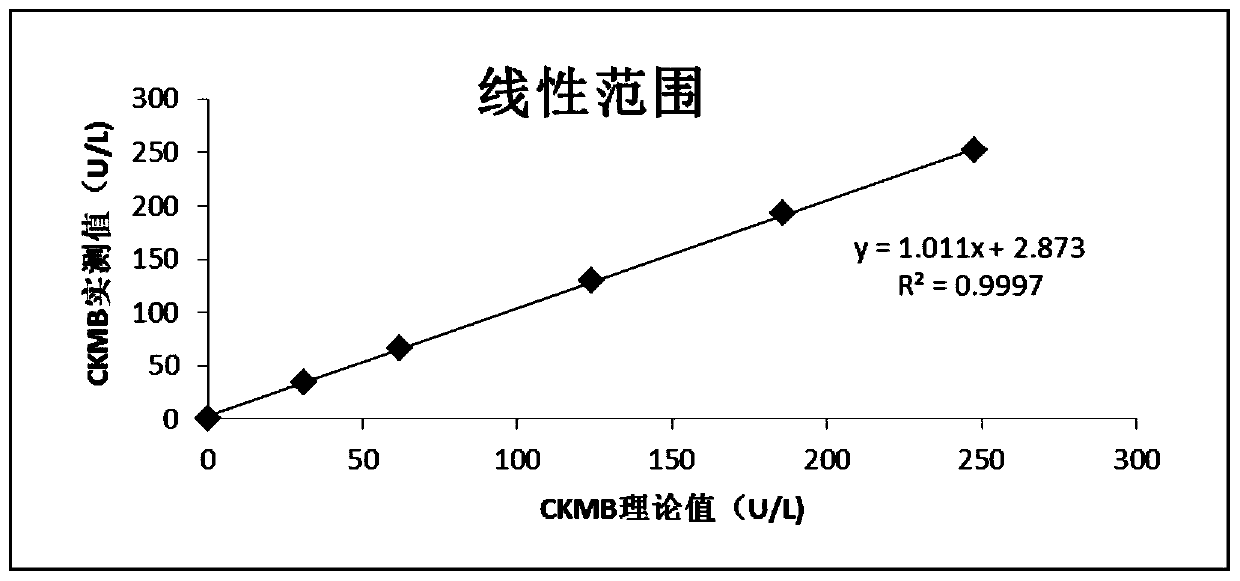
Kit for detecting glucose by hexokinase method and preparation method
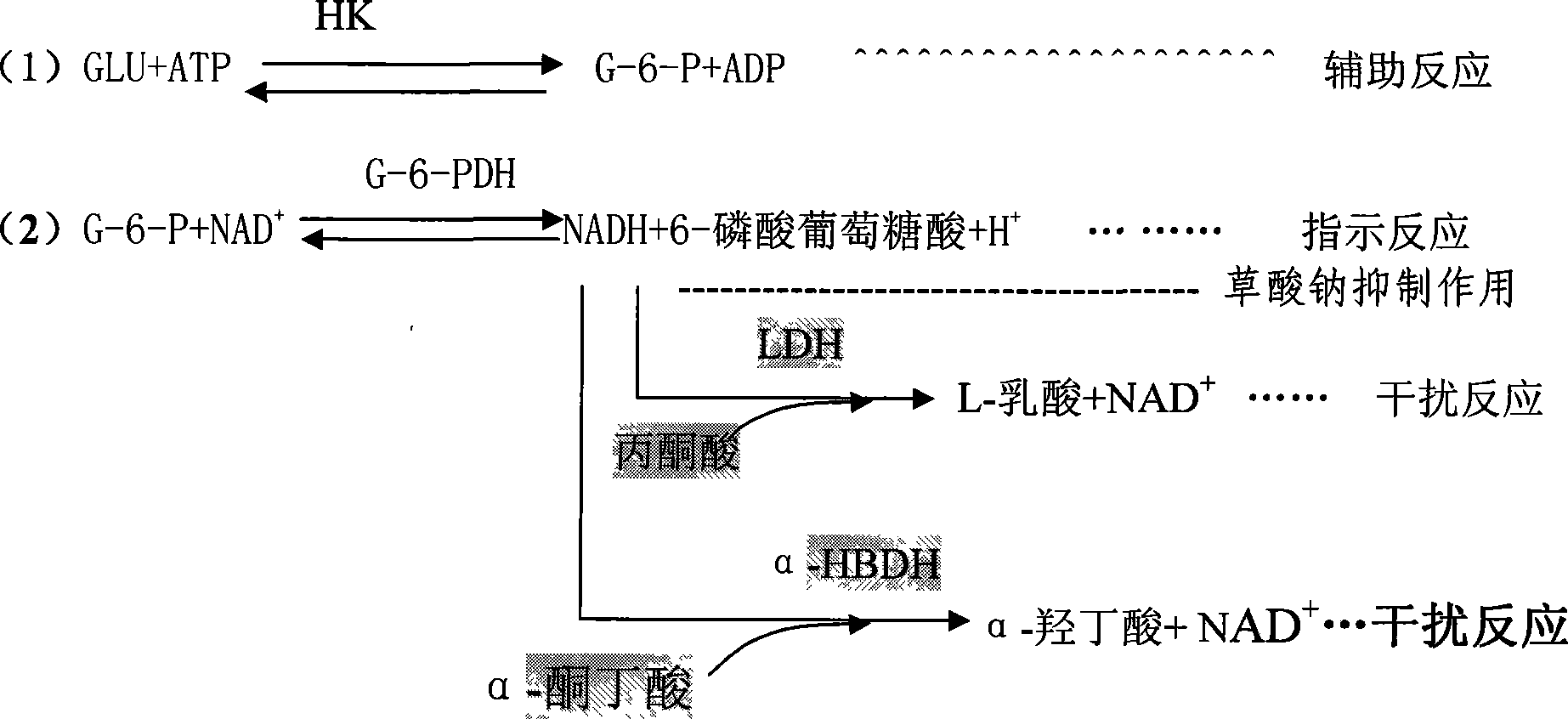
InactiveCN101386882AImprove accuracyLow costMicrobiological testing/measurementOxalateGlucose detection
The invention discloses a reagent kit for detecting glucose by a hexokinase method, which belongs to a reagent kit in a method for detection glucose containing enzyme. The reagent kit comprises four bottles of reagent I, one bottle of reagent II, and one glucose standard liquid, wherein the reagent I comprises oxalate with the concentration of 8.0mmol / L. By inhibiting the activities of endogenous lactate dehydrogenase and alpha-hydroxybutyrate dehydrogenase through the oxalate, the reagent kit controls an interference reaction, increases the specificity of the reaction, and improves the accuracy of detection results. The reagent kit is slightly affected by the volume ratio of sample reagents and the reaction time during the detection, and the linear range can reach 37.4mmol / L. A use method and the range of the reagent kit are the same as those of the prior hexokinase method; and the reagent kit can not increase the burden of experimental personnel, almost does not increase the cost of reagents, is economical, convenient and easy, and is the reagent kit which detects the glucose by the hexokinase method with higher accuracy.
Owner:TIANJIN BAODI HOSPITAL
Carbon dioxide kit employing enzymatic cycling assay
The invention discloses a carbon dioxide kit employing enzymatic cycling assay. The kit is composed of a NADH analogue cyclic regeneration system and a carbon dioxide test reagent, wherein the NADH analogue cyclic regeneration system consists of 6-phosphogluconate dehydrogenase, hexokinase, glucose and adenosine triphosphate disodium. The carbon dioxide kit is simple to prepare and low in cost, and the NADH analogue cyclic regeneration system in the kit can prolong the storage time of the kit and effectively improves the stability, accuracy and precision of testing.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Separation and purification method for Amanita virosa antibacterial active ingredient
InactiveCN101376894AGrowth inhibitionInhibition of germinationBiocideFungiATPaseCellular respiration
The invention relates to a separation and purification method of a Basidiomycetes antifungal component; the antifungal component is composed of two amide-type substances, and the molecular weight of the two substances is 5732.39 and 7187.01; the separation and purification process comprises the following steps: Basidiomycetes entity authentication, strain separation and purification, strain identification, mycelium liquid culture, antifungal component extraction, antifungal component separation, antifungal component purification. The method can inhibit chrysosperma mycelium growth and spore germination with 100 percent of inhibition rate, and has obvious inhibiting function to Pinus sylvestris germ at the same time. The Basidiomycetes antifungal substance affects the activity of seven enzymes of pathogenicbacteria, reduces the activity of hexokinase, improves the activity of pyruvate kinase, succinic acid, lactate dehydrogenase and ATP enzyme, and speeds up the respiration of germs.
Owner:NORTHEAST FORESTRY UNIVERSITY +2
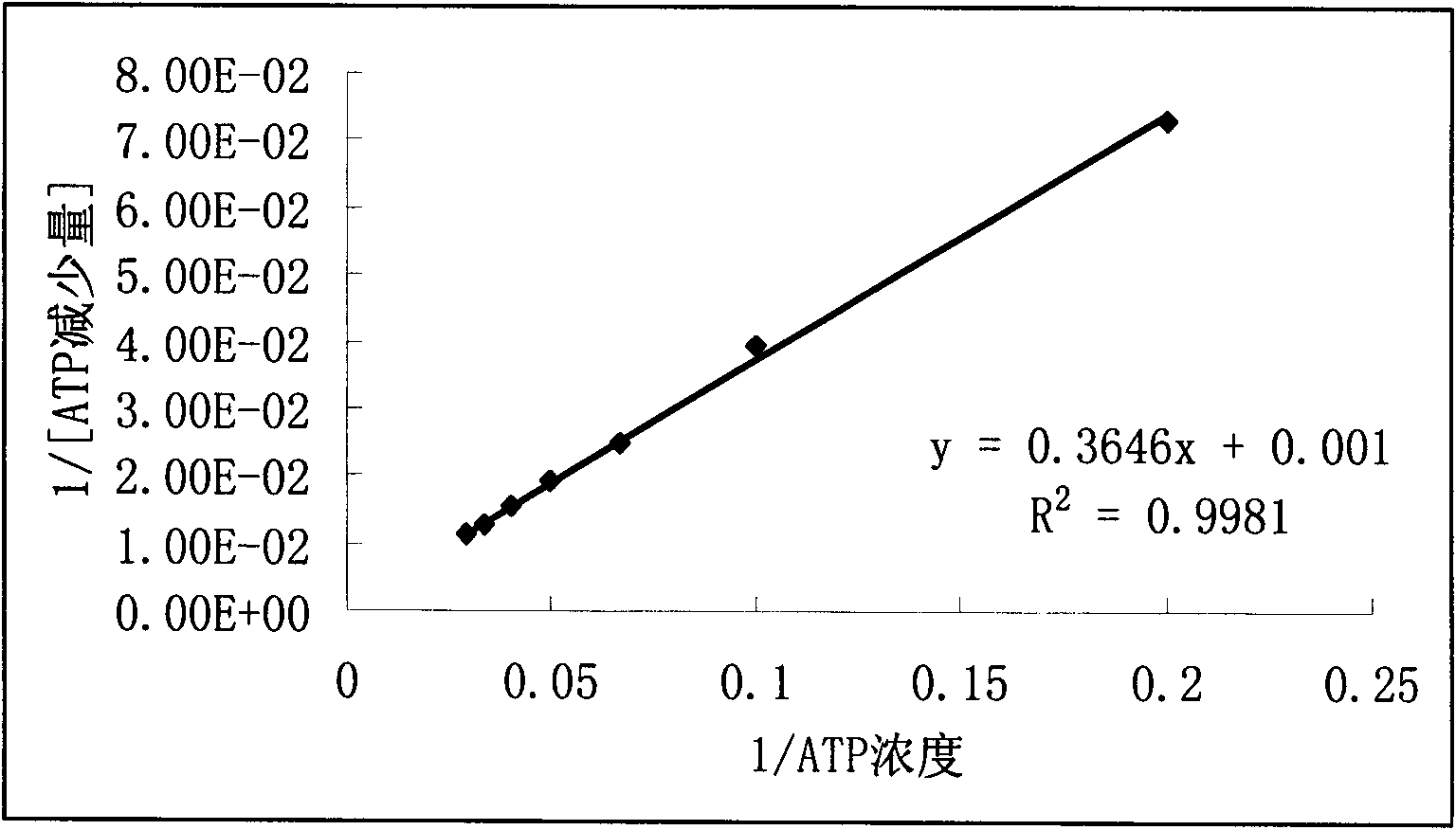
Method for screening active component with function of hexokinase inhibition effects
InactiveCN102335221AImprove MS signalImprove stabilityComponent separationAntineoplastic agentsScreening methodHexokinase
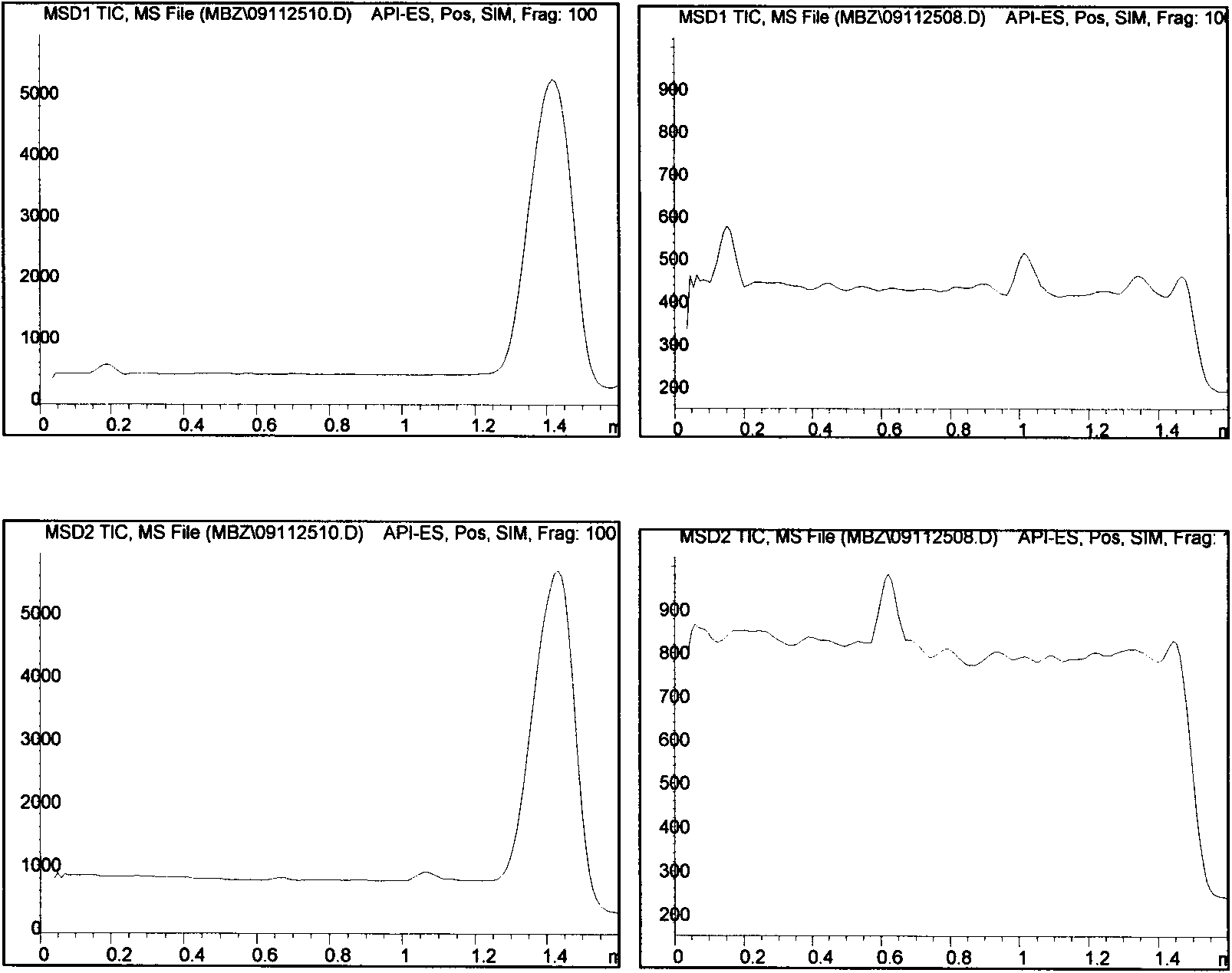
The invention belongs to the field of drug analysis, relates to a method for screening an active component in a traditional Chinese medicine, and specifically, relates to a method for screening a traditional Chinese medicine active component with a function of hexokinase inhibition effects. Based on hexokinase catalytic reaction adopting adenosine triphosphate (ATP) as a substrate, the method can screen out a traditional Chinese medicine active component with a function of hexokinase inhibition effects through quantitative analysis processes on ATP and product ATP. The method is rapid, simple, economic and effective. Compared with a cell test method, the method reduces an extract use amount, saves biochemical reagents, and shortens a screening period. Therefore, the method has great importance for screening and research of traditional Chinese medicine active components. In the invention, all experiment parameters of an improved liquid chromatography-mass spectrometry (LC-MS) method have good reference values for mass spectrometry technology-based determination of other biomolecules with similar properties.
Owner:FUDAN UNIV
Sodium diagnosis/measuring reagent kit and method for measuring sodium concentration
InactiveCN101173930AFast measurementImprove accuracyMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPhosphateHexokinase
The invention relates to a natrium diagnosis / determination reagent kit which utilizing a enzyme colorimetry method and an enzyme-linked method technology, simultaneously the invention also relates to a method principle for determining the natrium concentration, the composition and the components of a reagent, belonging to the technical field of the medicine / food / environment examination and determination. The main components of the reagent kit of the invention comprise buffer solution, coenzyme, deoxycholate citrate lactose sacharose agar, adenosine triphosphate, magnesium chloride, Beta-galactosidase, hexokinase, dextrose-6-phosphate dehydrogenase and stabilizer. A sample is mixed with the reagent according to a certain volume to lead to perform the enzymatic reaction, a reacting substance is put under an ultraviolet / visible light analyzer to examine the rising speed of absorbance at the position of a 340nm dominant wavelength, thereby detecting and calculating the natrium concentration value. Through the adoption of the invention, the required determined results can be obtained completely through the ultraviolet / visible light analyzer.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Creatine kinase isozyme bi-reagent and preparation method thereof
ActiveCN107641642AGood storage stabilityThe deviation of the measured value is smallMicrobiological testing/measurementCreatine kinaseSodium ascorbate
The invention mainly aims at providing a creatine kinase isozyme bi-reagent and a preparation method thereof. The reagent I is prepared from the following components: 90 to 120mmol / L of imidazole buffer solution, 15 to 25mmol / L of N-acetylcysteine, 25 to 35mmol / L of phosphocreatine, 1 to 10mmol / L of adenosine diphosphate, 1 to 10mmol / L of triphosadenine, greater than 1.5KU / L of glucose-6-phosphatedehydrogenase, greater than 2.5KU / L of heterophosphatase and greater than 2.0KU / L of creatine kinase isozyme antibody; 0.01 to 0.05 percent by mass concentration of polyvinylpyrrolidone, 0.01 to 0.05percent by mass concentration of tris(nonylphenyl) phosphate, 0.01 to 0.02 percent by mass concentration of lauryl dihydroxyethyl amine oxide, 0.05 percent of isomeric sodium ascorbate and the balance of water; the reagent II is prepared from the following components: by adopting a reagent II solution as a basis, 1 to 5mmol / L of nicotinamide adenine dinucleotide phosphate, 10 to 30mmol / L of glucose and the balance of water. In the case of storing for 14 days at 37 DEG C, the reagent stability is still relatively high, and measurement value deviation of the reagent is less than 10 percent.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Creatine kinase isoenzyme detection reagent kit
InactiveCN109837324AImprove stabilityHigh precisionMicrobiological testing/measurementCreatine kinaseCreatine kinase isoenzyme
The invention is suitable for the technical field of medical inspection, and provides a creatine kinase isoenzyme detection reagent kit. The reagent kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises the following components of 10-20g / L of biologic buffering agents, 1-3g / L of magnesium acetate, 5-10g / L of N-broncholysin, 0.5-2KU / L of hexokinase, 0.5-2.5g / L of a surfactant and 0.8-2.5mL / L of creatine kinase isoenzyme antibodies, and the reagent R2 comprises the following components of 5-20g / L of creatine phosphate, 1.5-5g / L of adenosine diphosphate and 8-18KU / L of glucose-6-phosphate dehydrogenase. The reagent kit disclosed by the invention can be applied to detection of the activity of the creatine kinase isoenzymes in serum of human bodies, the reagent stabilityis high, and the detection result is high in precision and accuracy.
Owner:曾宪亮
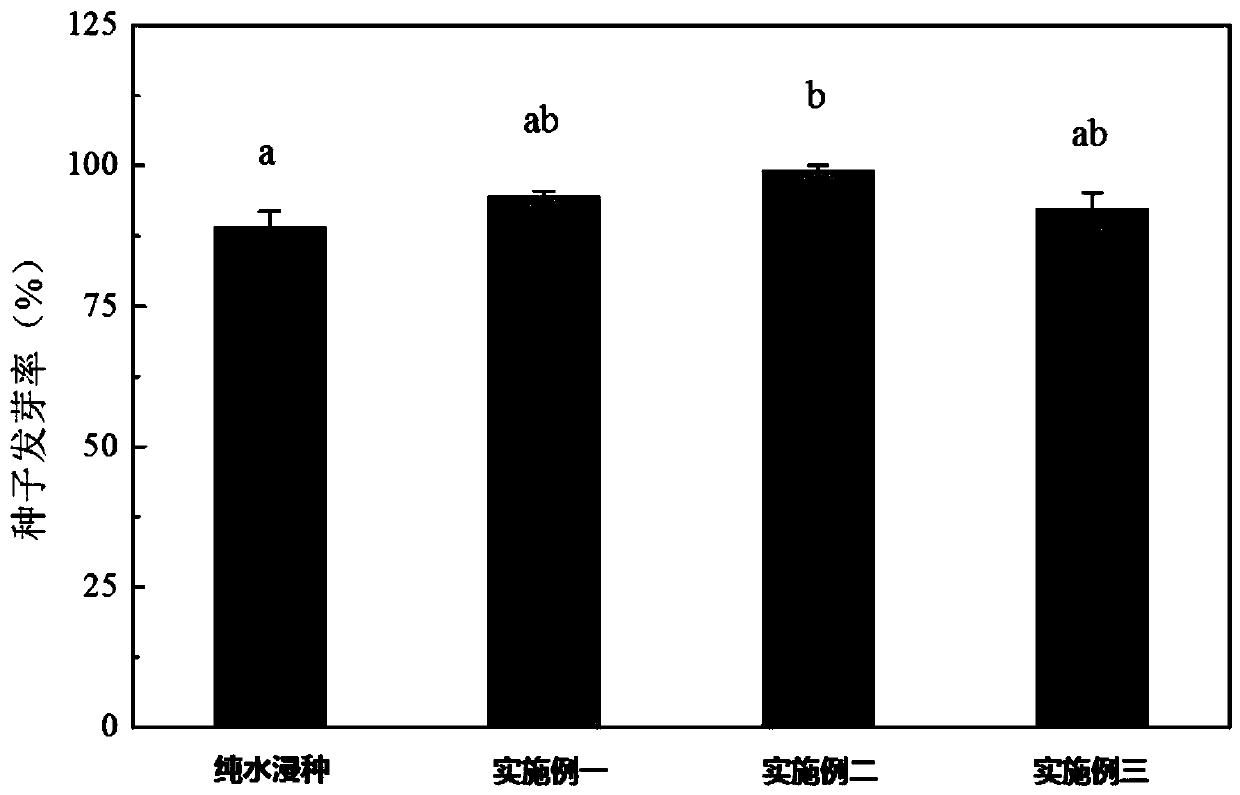
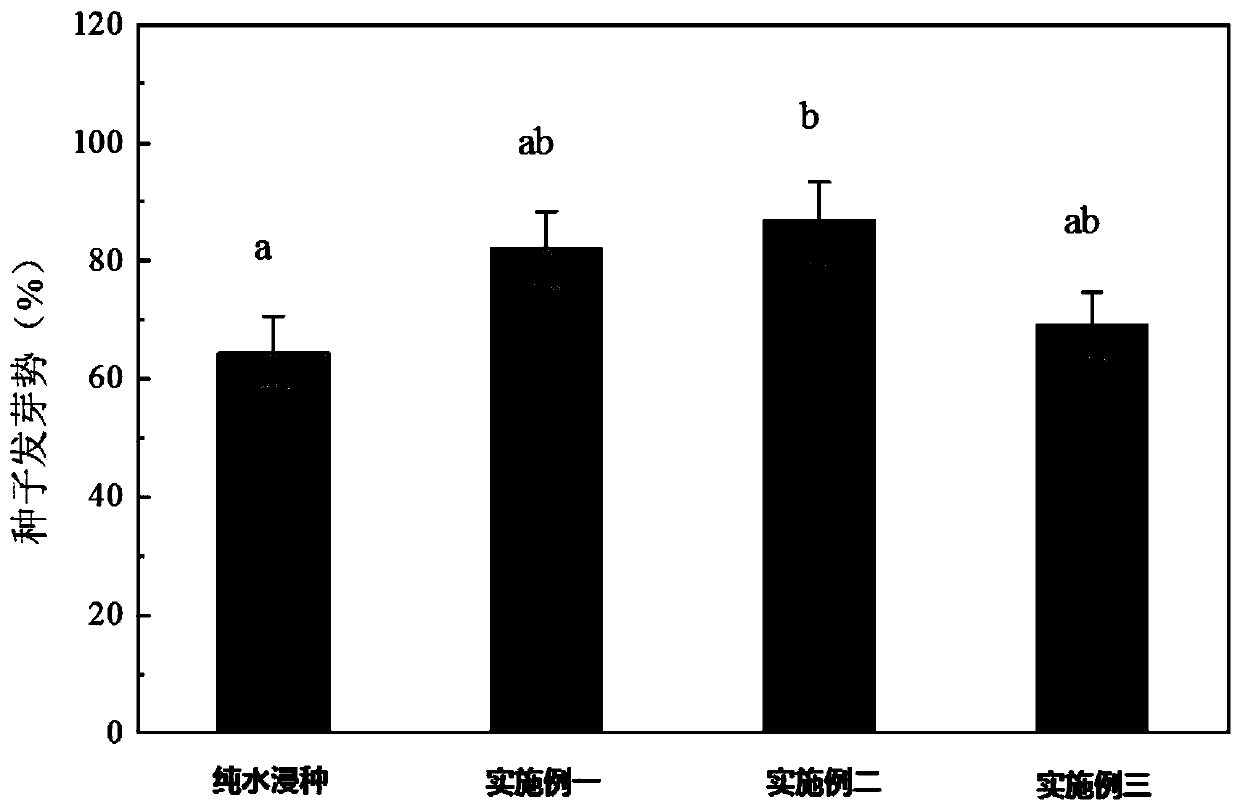
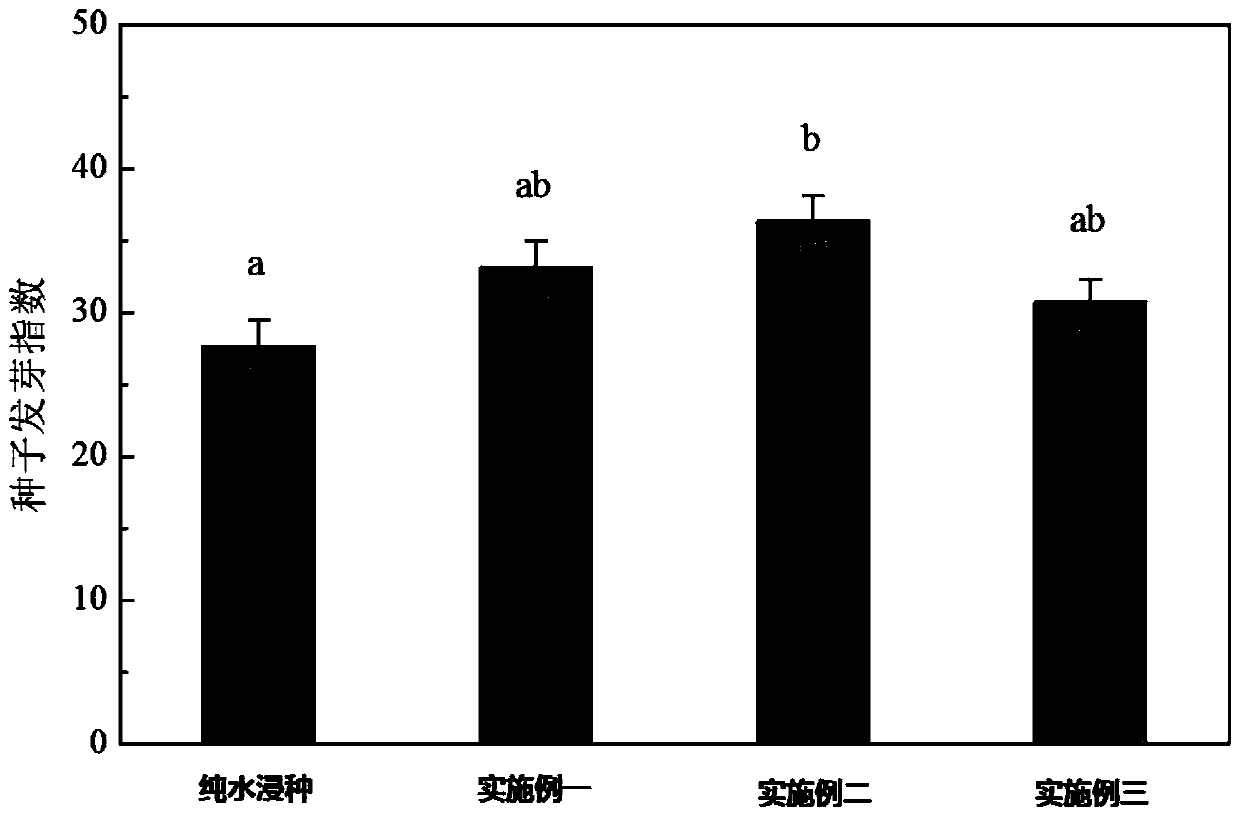
Method for improving germination and growth of corn seeds
InactiveCN110337857AShorten the timeImprove germination rateCereal cultivationSeed immunisationUridine diphosphate glucose pyrophosphorylaseMetabolite
The invention discloses a method for improving germination and growth of corn seeds and relates to the field of crop planting. The method aims to solve the technical problems of low germination rate and long germination period of existing corn seeds. The method comprises the steps of 1, sterilizing the surfaces of the seeds; 2, preparing a seed soaking agent; 3, performing vibrated seed soaking treatment. The method can shorten the average time of seed germination and increase the germination rate, germination vigor, germination index and vigor index of the seeds. The growth and elongation ofa root system and germs are boosted; the activity of hexokinase and uridine diphosphate glucose pyrophosphorylase is obviously increased, the activity of aldolase is reduced, the activity of phosphofructokinase is maintained at a relatively normal level, the glycolysis metabolism and the biosynthesis process of cell walls are promoted, and metabolic products and energy are provided for the germination and growth of the seeds. The method is used for promoting the germination and growth of the corn seeds.
Owner:NORTHEAST INST OF GEOGRAPHY & AGRIECOLOGY C A S
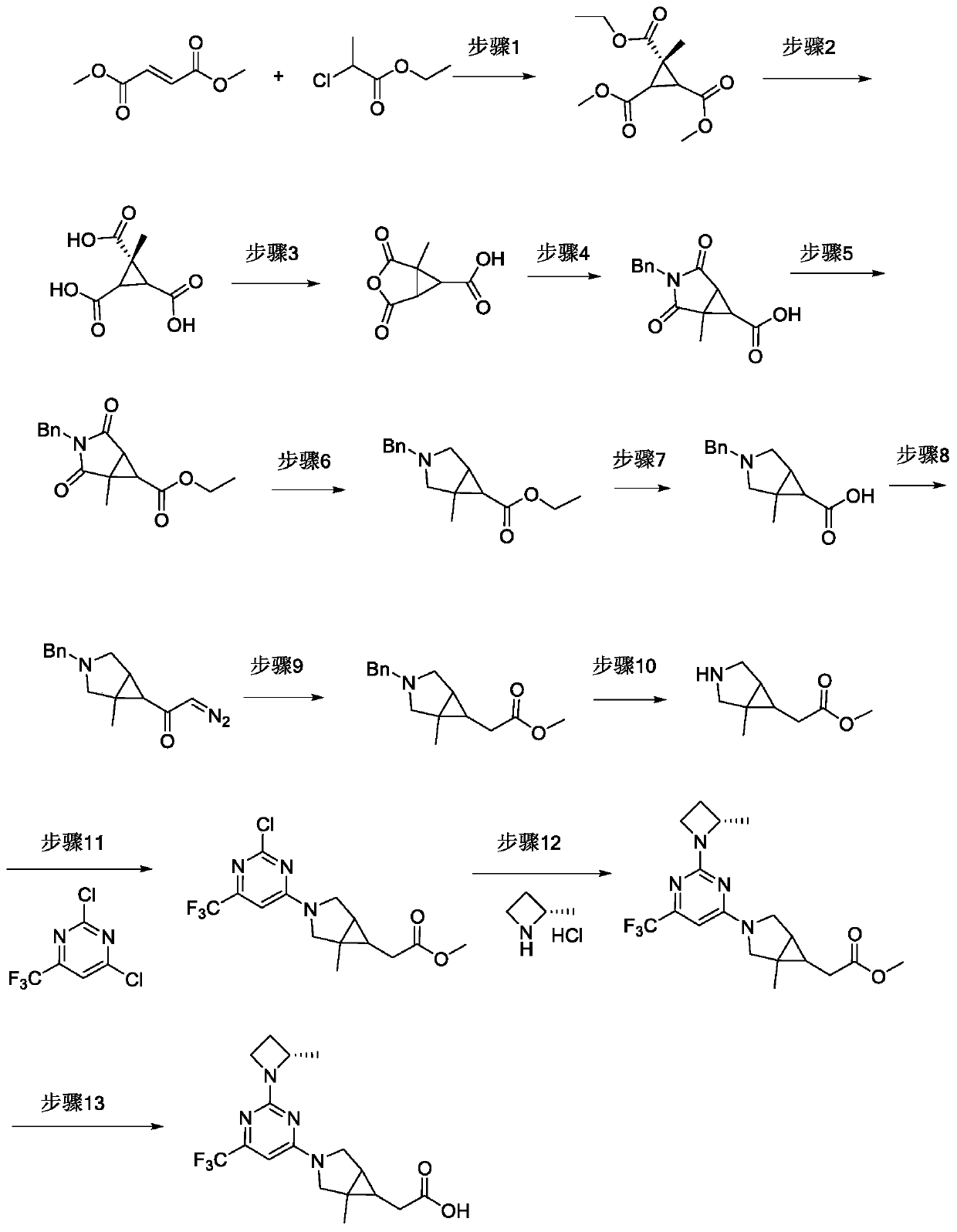
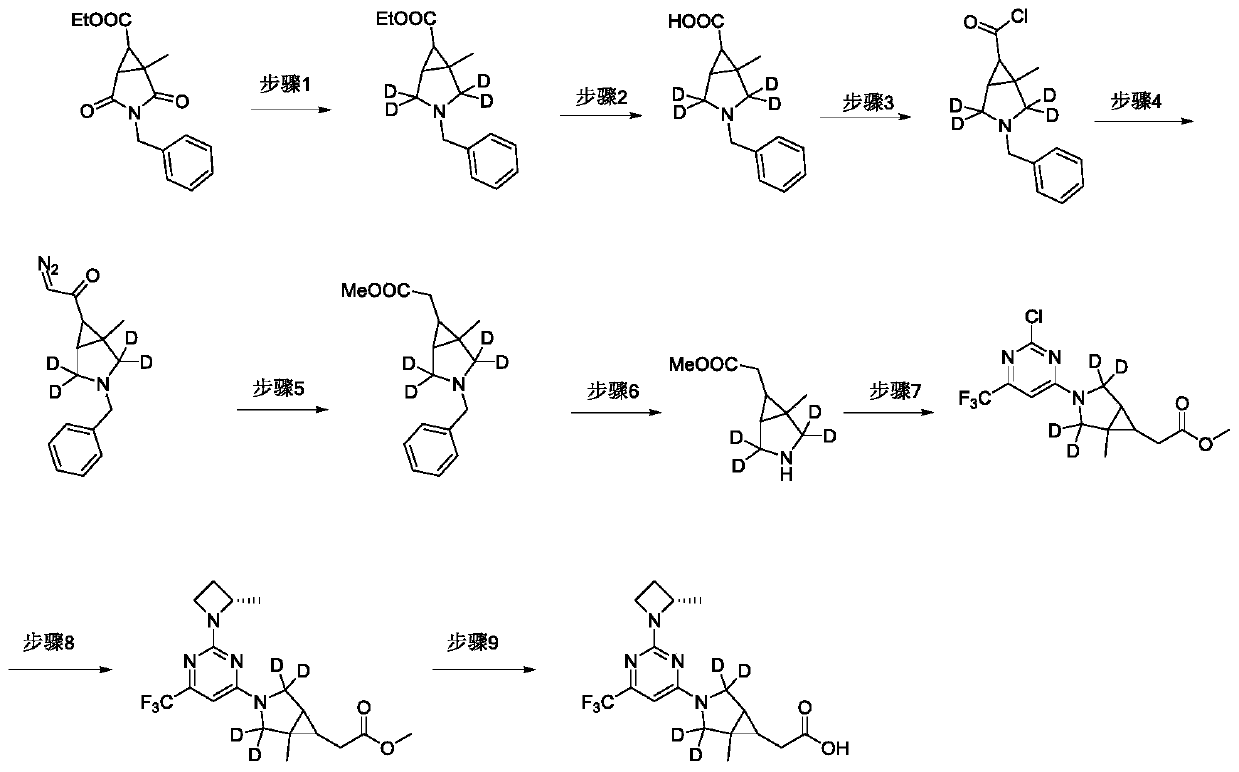
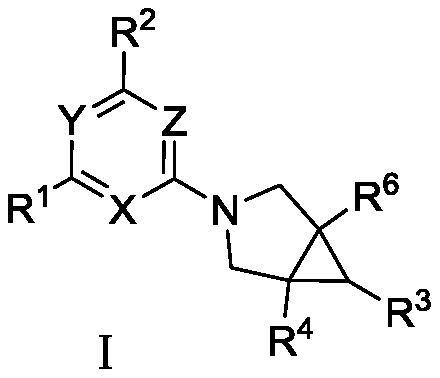
Fused ring compound used as hexosylkinase inhibitor
InactiveCN111423420AEnhanced inhibitory effectOrganic active ingredientsSenses disorderDiseaseDiabetes mellitus
The invention provides a compound or a pharmaceutically acceptable salt thereof or a stereoisomer or isotope labeled compound thereof. The compound has a good inhibition effect on hexokinase. Meanwhile, the invention also provides a pharmaceutical composition containing the compound or the pharmaceutically acceptable salt thereof or the stereoisomer or isotope labeled compound thereof. The invention further discloses an application of the compound or the pharmaceutically acceptable salt or the stereoisomer or isotope labeled compound in preparation of drugs for treating diseases such as T1D, T2D, LADA, EOD, YOAD, MODY, dystrophy related diabetes and gestational diabetes.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com