Fused ring compound used as hexosylkinase inhibitor
A compound and heterocyclic technology, applied in the field of hexacyclic compounds as hexokinase inhibitors, can solve problems such as inflammation, liver fibrosis, and even liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
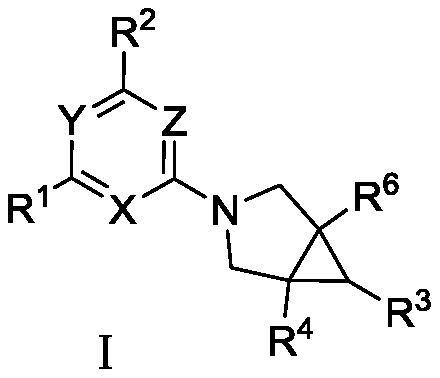
[0079] 2-(1-methyl-3-(2-((S)-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidin-4-yl)-3- The structural formula of azabicyclo[3.1.0]hexan-6-yl)acetic acid is as follows:
[0080]
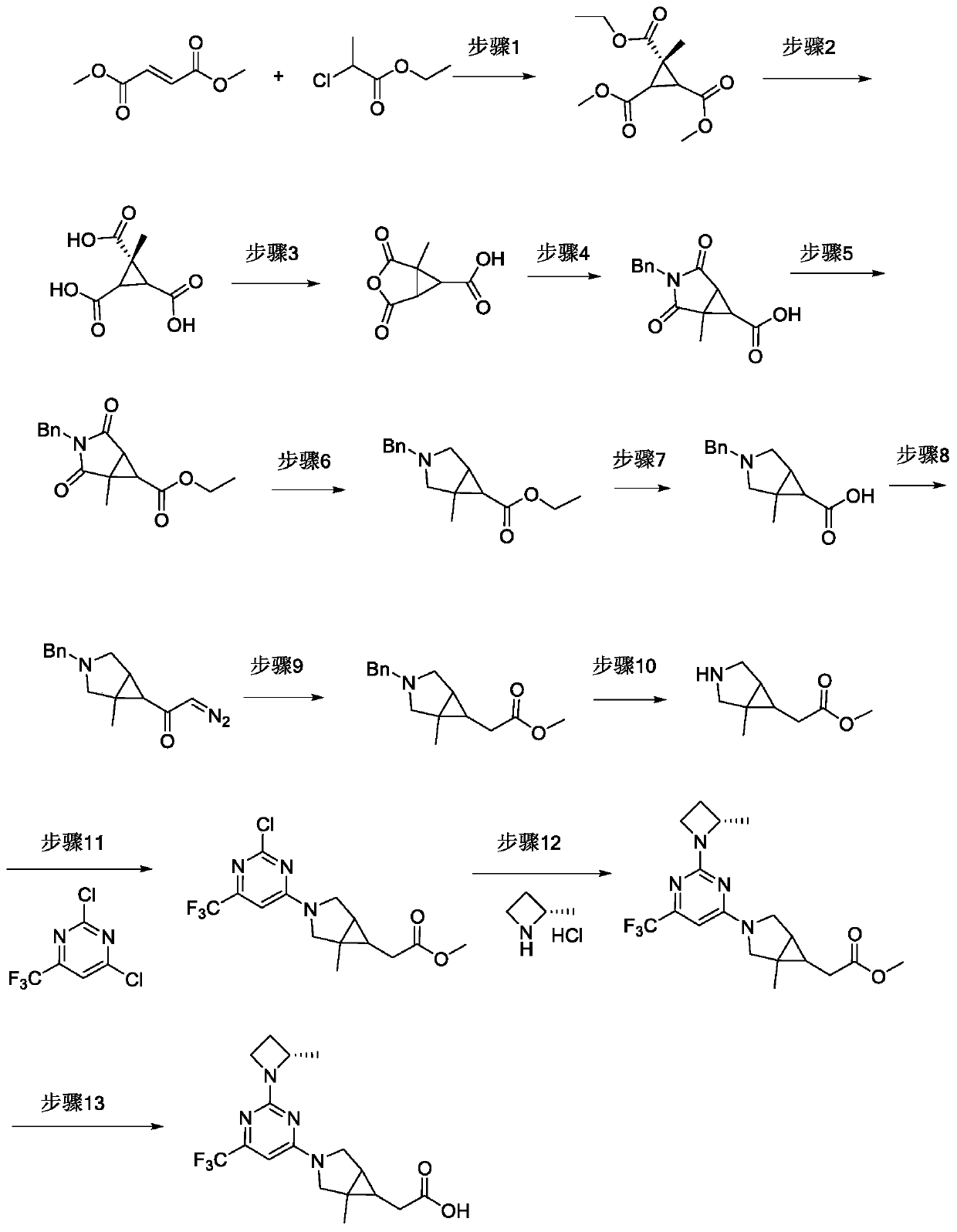
[0081] The synthetic route diagram of the compound described in this embodiment is as attached figure 1 As shown, the specific preparation method of the compound described in this embodiment includes:
[0082]Step 1: Ethyl 1-methylcyclopropane-1-carboxylate-2,3-methylcarboxylate
[0083] At room temperature, add dimethyl fumarate (10 g, 69.38 mmol) and benzyltriethylammonium chloride (0.16 g, 0.69 mmol) to a solution of NaH (2.16 g, 90.2 mmol) in DMF (100 mL), slowly Ethyl 2-chloropropionate (10.42 g, 76.32 mmol) was added dropwise, and the reaction was stirred overnight at 40°C.
[0084] Pour the reaction solution into ice water (30 mL), extract with methyl tert-butyl ether (30 mL×2), combine the organic phases, wash with saturated brine (80 mL), dry over anhydrous sodium sulfate, filter, an...
Embodiment 2
[0117] 2-(1-methyl-3-(2-((S)-2-methylazetidin-1-yl)-6-(trifluoromethyl)pyrimidin-4-yl)-3- Azabicyclo[3.1.0]hexan-6-yl-2,2,4,4-d 4 ) acetic acid
[0118] The structural formula is as follows:
[0119]
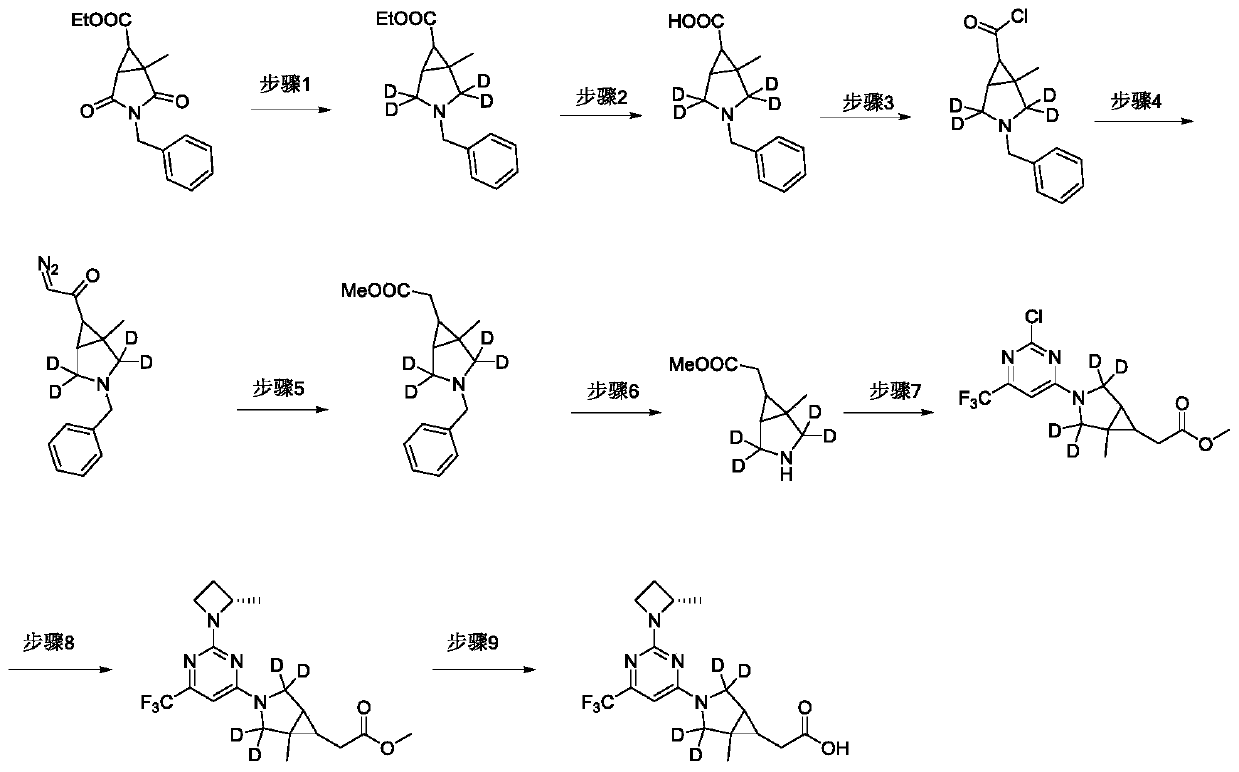
[0120] The synthetic route diagram of the compound described in this embodiment is as attached figure 2 As shown, the specific preparation method of the compound described in this embodiment includes:
[0121] Step 1: 3-Benzyl-1-methyl-3-azabicyclo[3.1.0]hexane-6-carboxylic acid ethyl ester-2,2,4,4-d 4
[0122] At 0°C, slowly drop boron trifluoride diethyl ether (22.10 g, 73.18 mmol, 47%) into a solution of sodium borodeuteride (2.3 g, 54.89 mmol) in tetrahydrofuran (50 mL). Benzyl-1-methyl-2,4-dioxo-3-azabicyclo[3.1.0]hexane-6-carboxylic acid ethyl ester (5.0g, 18.03mmol) in tetrahydrofuran (30mL) solution, dropwise , stirred overnight at room temperature. The reaction solution was cooled in an ice bath, and then slowly added dropwise ethanol (50 mL). Part of the so...
Embodiment A
[0144] Embodiment A: in vitro enzymatic activity experiment
[0145] There are two isoforms of ketohexokinase, KHKc and KHKa, among which KHKc has more than ten times the ability to phosphorylate fructose than KHKa.
[0146] According to the metabolic pathway of fructose, the enzyme function of KHK is to consume ATP to convert fructose into fructose-1-phosphate and produce ADP at the same time. The consumption of NADH and the final product NAD can be detected by ATP depletion method, ADP chromogenic method, or by subsequent reactions + The generation of , can effectively measure the inhibition of the reaction.
[0147] The content of NADH was monitored by continuously measuring the absorbance at 340nm, and the inhibition of the candidate compound to KHK was preliminarily judged according to the consumption of NADH. The method involves a coupled enzyme system, and the 3-step reactions involved are as follows:
[0148]
[0149] 3-step reaction involved in initial screening...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com