Method for synthesizing of 2'-fucosyllactose
A technology of fucosyllactose and fucosyl, which is applied in the field of genetic engineering, can solve the problems of expensive glycoside donors, complex synthetic routes, and inability to achieve large-scale production, and achieve strong basic theoretical research value and social and economic benefits. The effect of reducing production costs and broad market development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Construction of recombinant Lactococcus lactis and recombinant Escherichia coli
[0067] The genes involved in the present invention can be obtained through genomic PCR amplification, or can be obtained through complete sequence synthesis according to the gene sequence. The following is an example of PCR amplification:
[0068] 1. Amplification of the target gene
[0069] (1) Extract the genomic DNA of Escherichia coli (E.coli K12), and design the following primers to amplify hexokinase gene glk, phosphomannose mutase gene manB, and mannose-1-phosphate guanylyltransferase in sequence Gene manC, GDP-mannose 4,6-dehydratase gene gmd, GDP-4-keto-6-deoxymannose 3,5-mutrotase / 4-reductase gene wcaG:
[0070]
[0071]
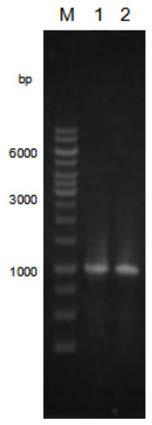
[0072] (2) Genomic DNA of Helicobacter pylori (Helicobacter pylori) HPAG1 was extracted, and the following primers were designed to amplify the α1,2-fucosyltransferase gene futc:
[0073] Primer name Sequence 5'-3' Restriction...
Embodiment 2
[0092] Example 2, Induced expression analysis of recombinant strains
[0093] 1. Induced expression analysis of recombinant Escherichia coli
[0094] (1) Pick the genetic engineering strain BL21 / pET-gmd that embodiment 1-3 obtains, BL21 / pET-wcaG, BL21 / pET-futc single bacterium colony transfers respectively in 5mL LB liquid medium, 37 ℃, 220r / min cultivated overnight;
[0095] (2) Transfer the bacterial solution to 50mL M9 liquid medium, and control the initial bacterial concentration OD 600 =0.1, continue to cultivate;
[0096] (3) When the strain concentration reaches OD 600 When the temperature is 0.4, add 40 μL of 0.1mol / L IPTG and induce at 16°C for 4 hours;
[0097] (4) Centrifuge at 6000r / min for 10min to collect Escherichia coli;
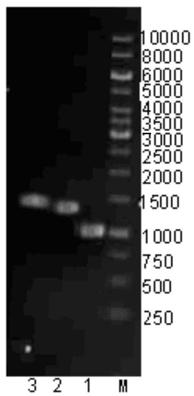
[0098] (5) Wash the strain twice with PBS buffer, break the cells with an ultrasonic cell breaker, centrifuge at 12000r / min for 10min at 4°C, collect the supernatant, and take a sample for SDS-PAGE analysis. The results are shown in F...
Embodiment 3
[0106] Example 3 Recombinant Engineering Strain Fermentation and Enzyme Co-catalyzed Synthesis of 2'-Fucosyllactose
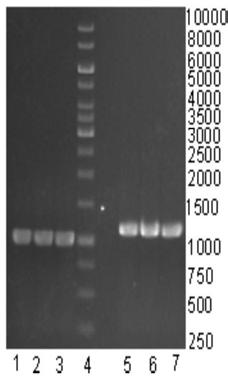
[0107] (1) Pick a single colony of recombinant Lactococcus lactis NZ3900 / pNZ-glk-manB-manC and inoculate it into 5mL GM 17 In liquid culture medium, cultivate overnight at 30-37°C, 160-220r / min. Inoculate the bacterial liquid into 50mL M9 fermentation medium, and control the initial bacterial concentration OD 600 = 0.1, the OD of the strain to be 600 When = 0.4, add 20 mg / mL of substrate mannose, and add Nisin as an inducer at the same time to make the final concentration 35 ng / mL, and ferment for 40 hours at 35°C and 200 r / min. Break the cells, centrifuge, and collect the supernatant;
[0108] (2) Preparation of GDP-mannose 4,6-dehydratase, GDP-4-keto-6-deoxymannose 3,5-mutrotase / 4-reductase and α1,2-fucosyltransferase : Pick single colonies of recombinant Escherichia coli BL21 / pET-glk, BL21 / pET-manB and BL21 / pET-manC and inoculate them into 5mL LB liquid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com