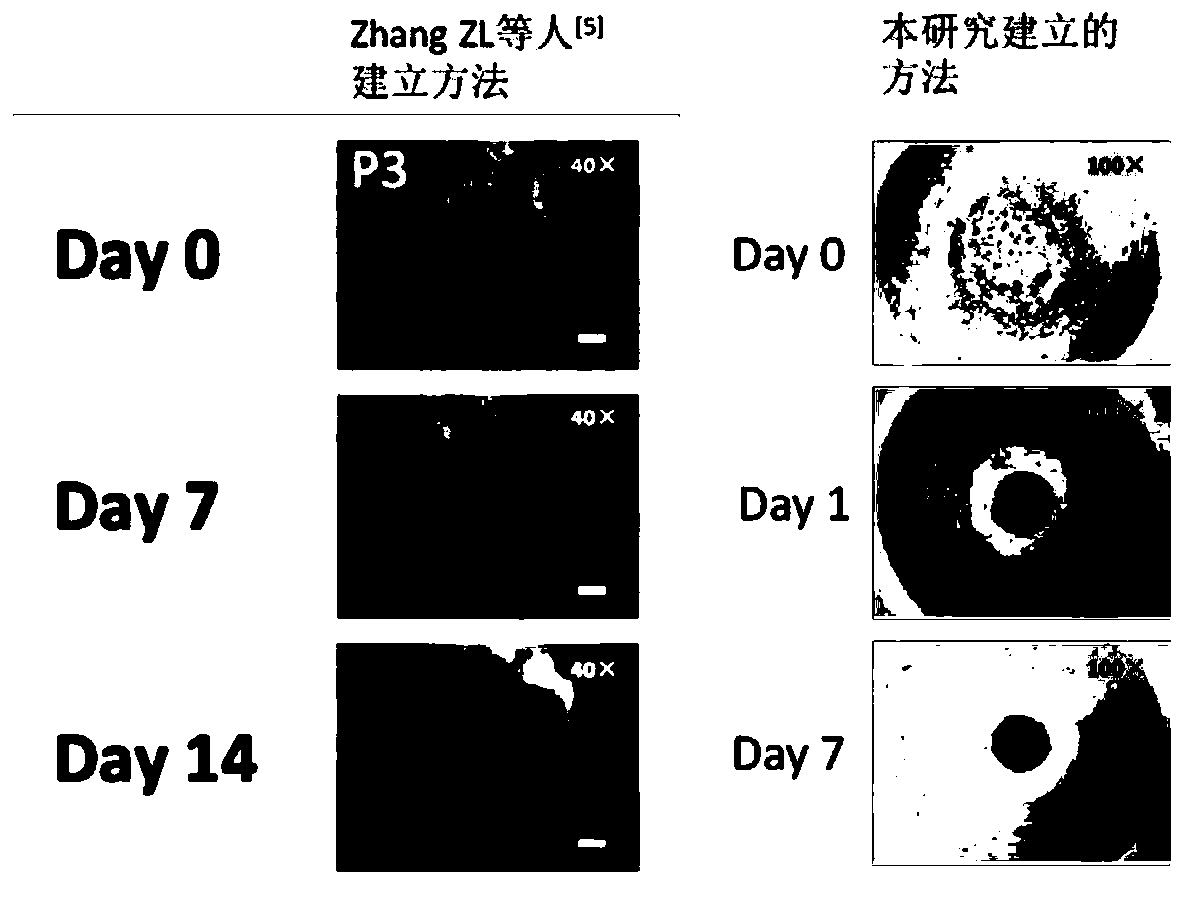
Specific culture medium for lung tumor organ and stentless 3D culturing method
A culture method and organoid technology, applied in 3D culture, culture process, tissue culture, etc., can solve problems such as poor culture effect, and achieve the effect of retaining heterogeneity, uniform and controllable size, and strong cell stemness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of lung cancer primary cell organoids
[0042] 1. Tumor tissue acquisition from lung cancer patients
[0043] The tissues used to isolate primary tumor cells are mainly derived from tumor tissue samples obtained by surgical resection or biopsy of lung cancer patients. Patients were required not to have received any anti-tumor therapy.
[0044] For surgically resected tumor specimens, samples should be taken from parts of the tumor that are actively growing and have no necrotic degeneration, and paracancerous tissues should be removed, because the paracancerous tissues contain fewer tumor cells and have poor cell viability, which reduces the survival rate of cells.
[0045] (1) Fresh tissue acquisition and processing methods:
[0046] Fresh tissue pieces removed by surgery or fresh tissue samples obtained by biopsy should be processed and separated immediately. Since the tissue has just left the human body, the shape, vitality and metabolic charac...
Embodiment 2
[0088] Example 2 Cryopreservation of Primary Tumor Cells
[0089] The fresh or cryopreserved and revived primary tumor cells in Example 1, if they do not need to be prepared into organoids temporarily, they can be cryopreserved when the number of adherent cells reaches more than 80% and the growth status is good.
[0090] Aspirate the serum-free medium, then add 1mL TrypLE cell digestion solution to each well, shake gently for 20s to make it evenly cover the bottom of the well; after 20s, suck the TrypLE cell digestion solution, put the 6-well plate in the incubator and incubate for 1 -2min; then observe the state of the cells under a microscope, and when it is found that there is obvious contraction, you can add 2mL serum-free medium to stop the digestion; gently blow with a pipette to make the cells fall off (minimize the generation of air bubbles as much as possible); then the cells Transfer the suspension to a 15mL centrifuge tube, centrifuge at 1000rpm / min for 5min, remov...
Embodiment 3
[0092] Example 3 Preparation of A549 cell organoids
[0093] Digest the A549 cells cultured to 80% confluence, count them, and inoculate them into 96-well ultra-low adsorption 96-well U-shaped culture plates according to a certain ratio. After the cells were inoculated, add the special medium in Table 2, and put it in 37°C, 5% CO 2 cultured in an incubator.
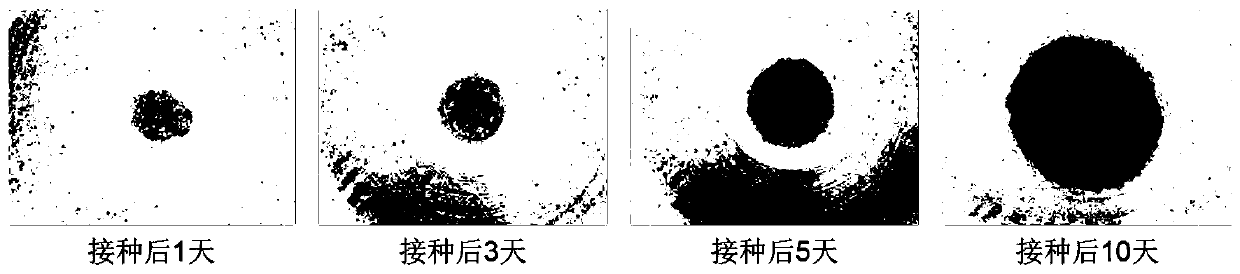
[0094] 30 minutes after inoculation, the cells aggregate due to gravity, and within 24 hours they can form organoid spheres. After that, the cell spheres begin to assemble and become more and more compact. After 5-6 days, they stabilize and begin to increase in size. The complete medium was replaced once a day.
[0095] image 3 The organoids formed for A549 cells and the morphological changes during the culture process. It can be seen from the figure that the cells have aggregated 30 minutes after inoculation, and the degree of aggregation increased 1 day after inoculation, and formed a relatively compact spherical or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com