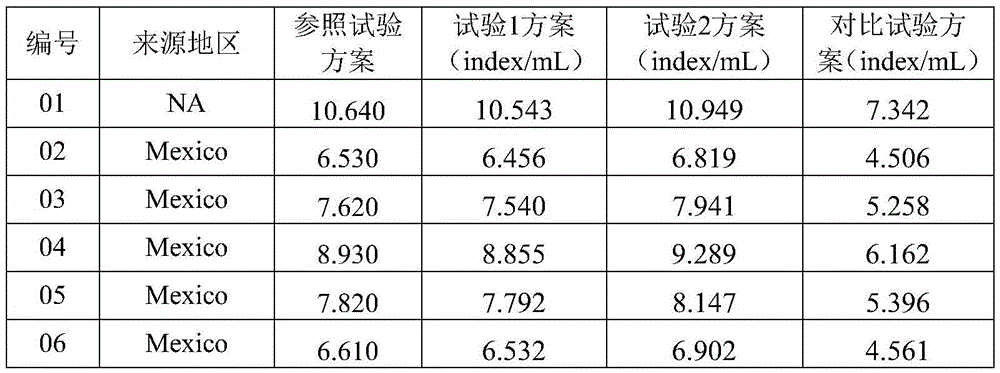
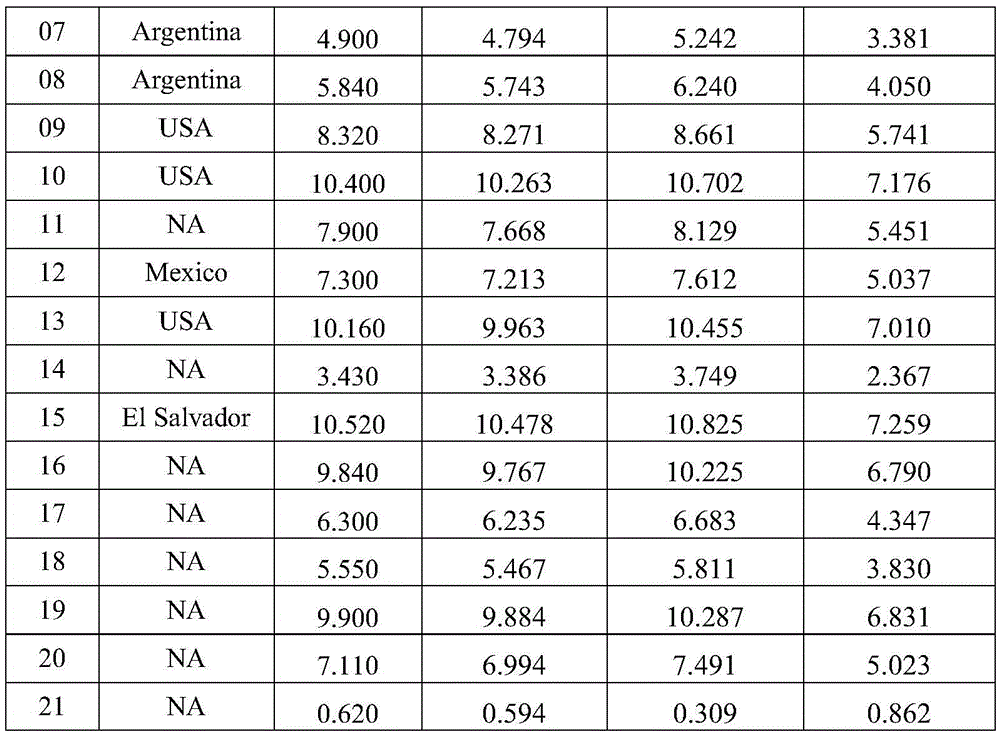
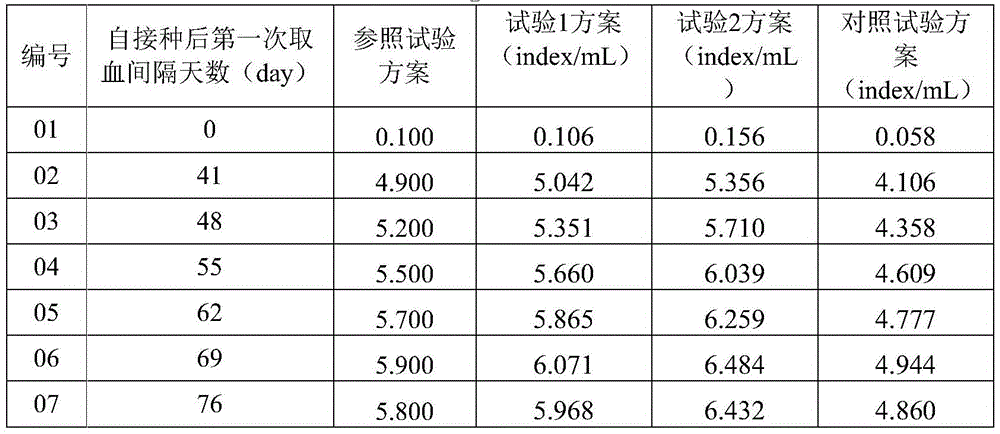
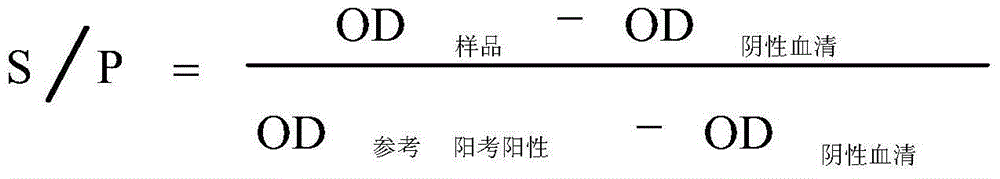
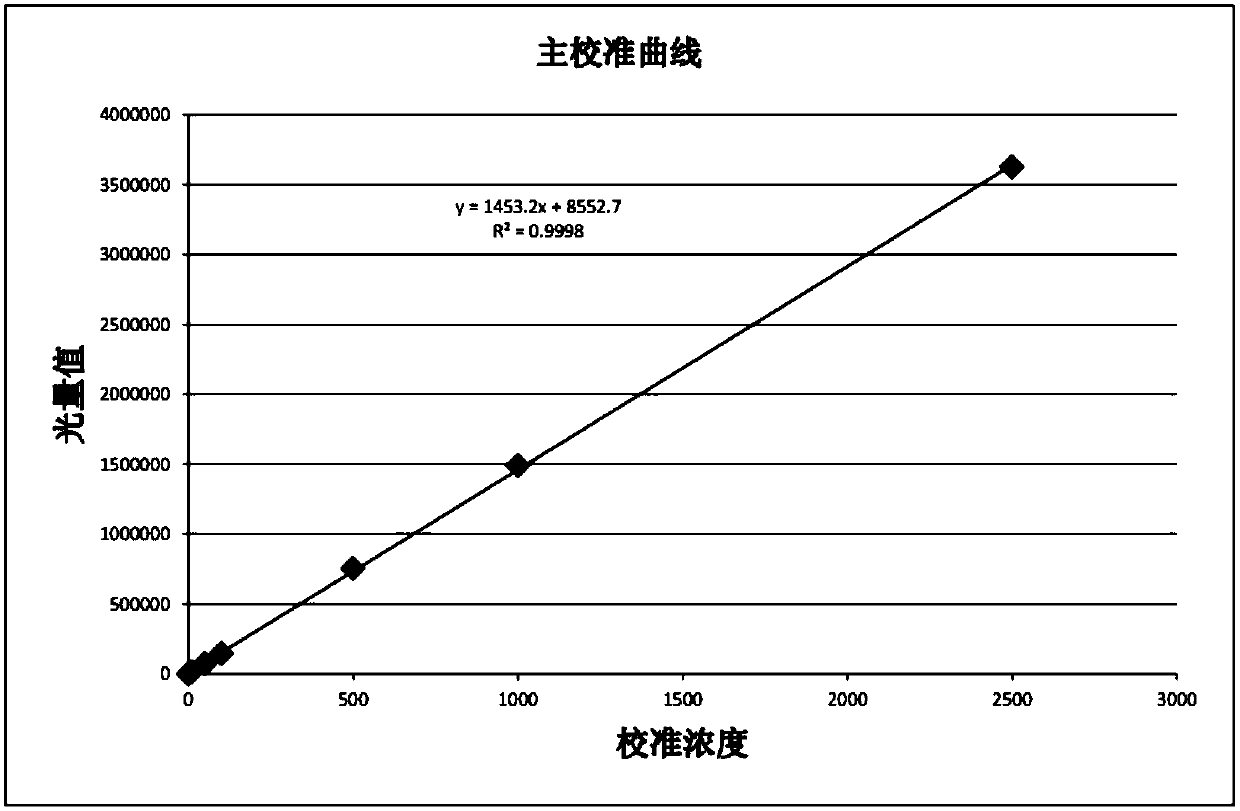
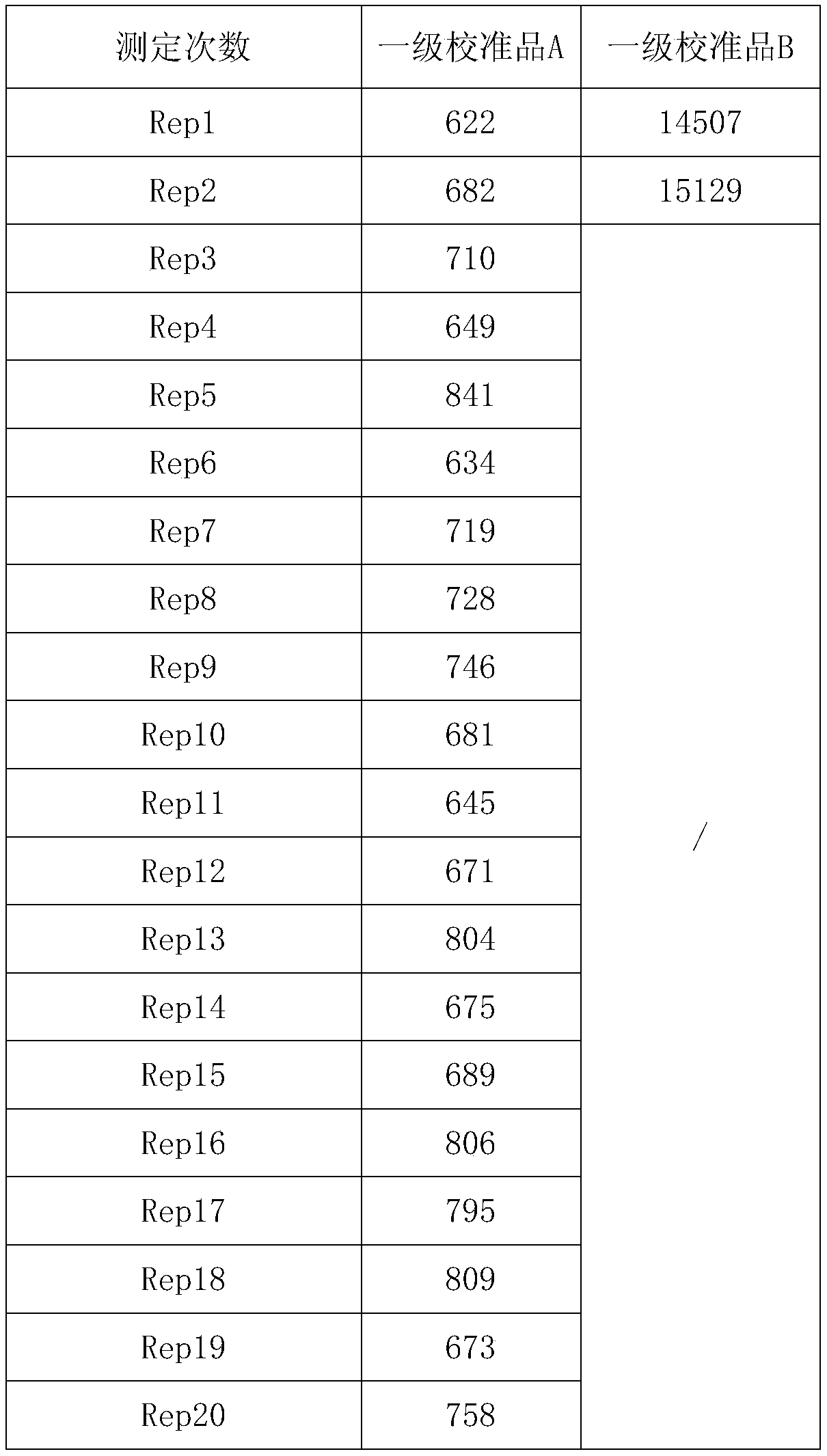
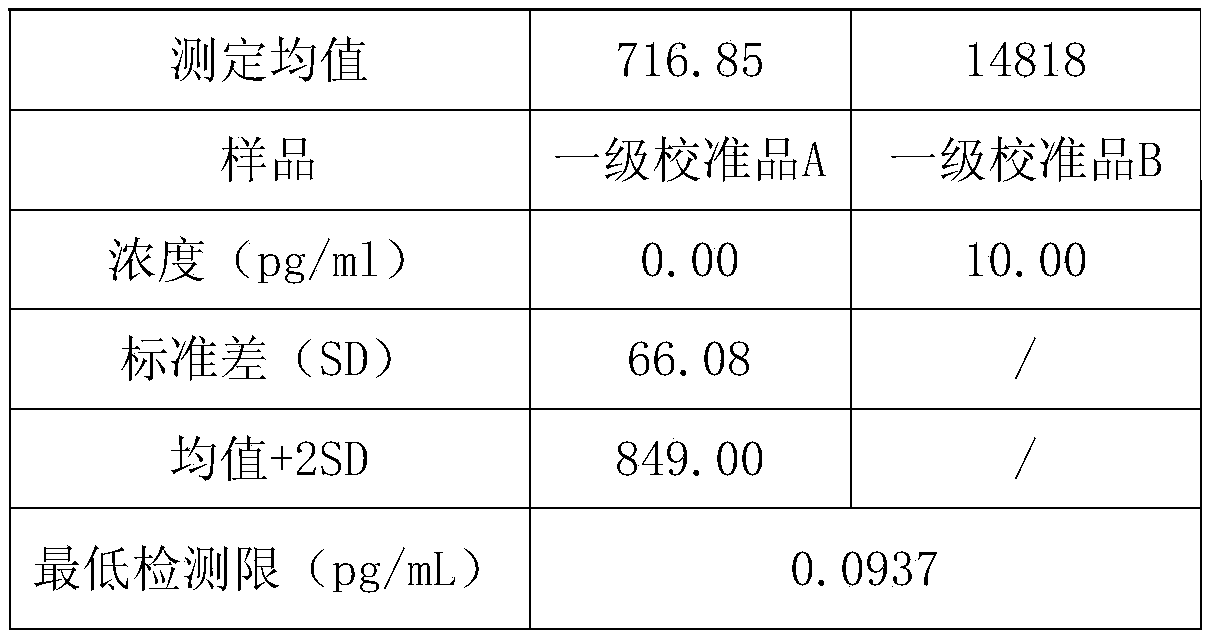
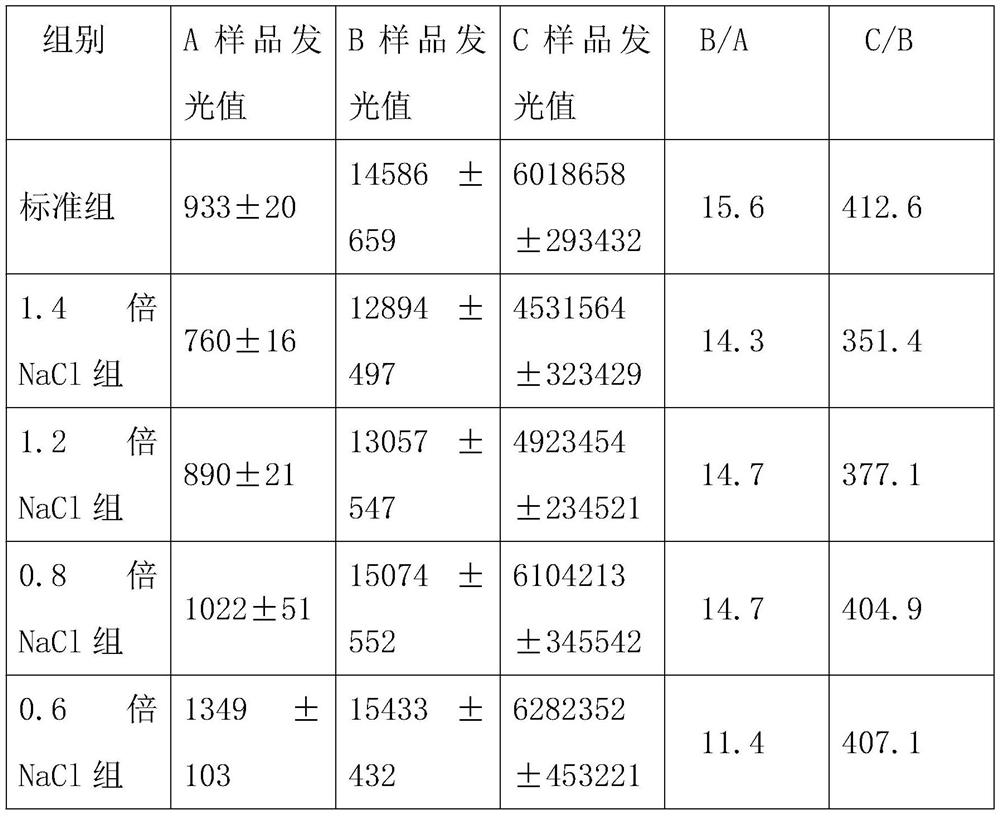
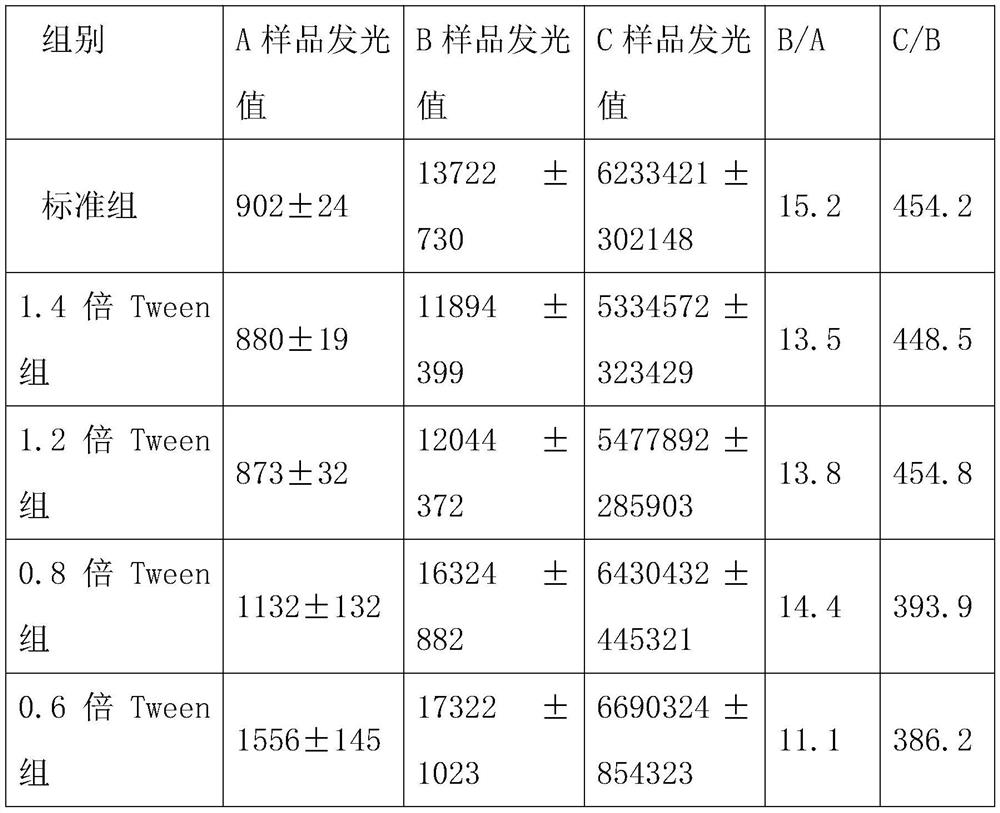
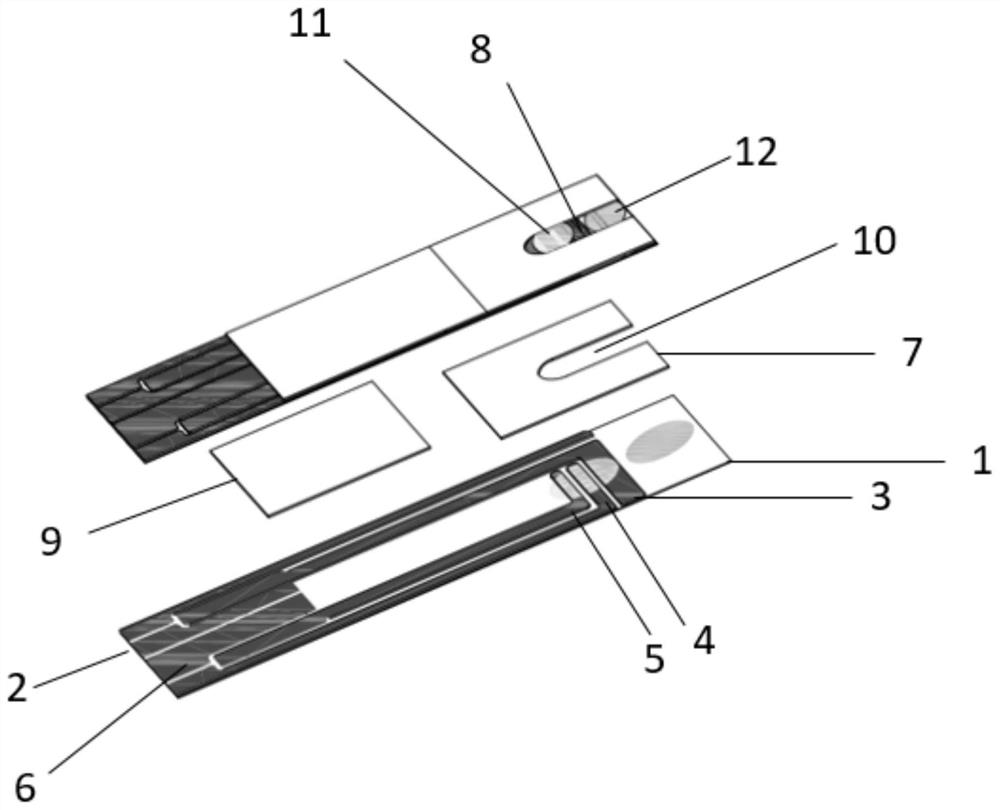
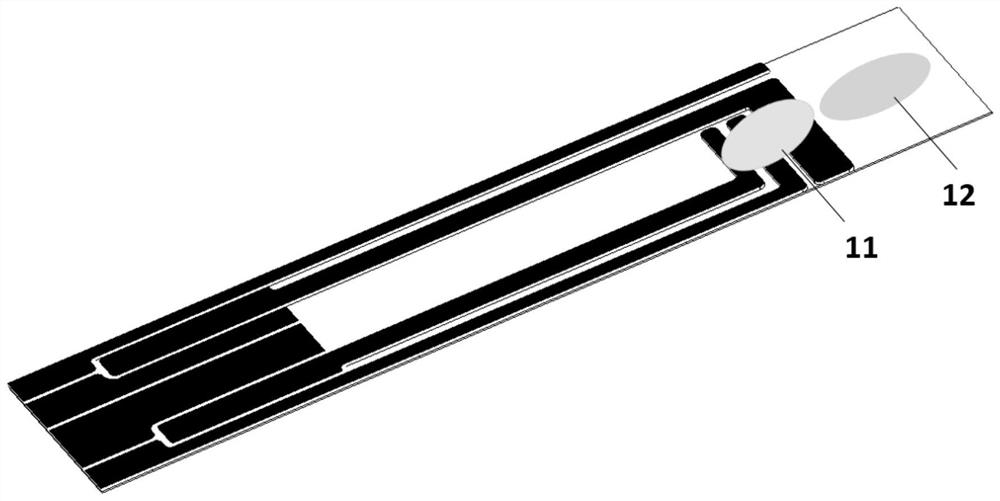
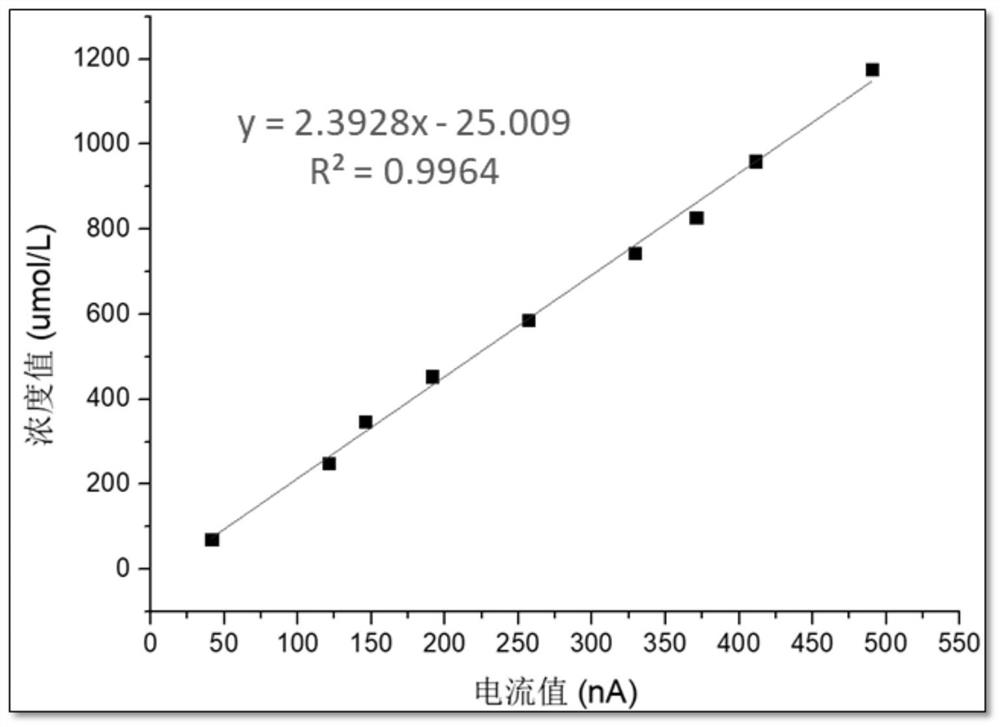
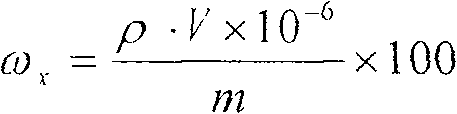Patents
Literature
88results about How to "Low background value" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kit for detecting trypanosoma cruzi antibody as well as preparation and application thereof
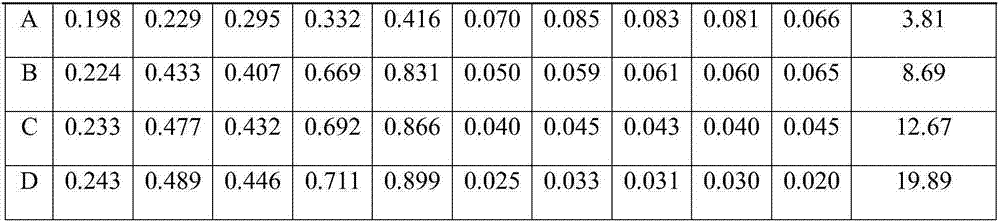
ActiveCN105548565AAccurate measurementSensitive assayChemiluminescene/bioluminescenceBiological testingAntigenMedicine
The invention provides a kit for detecting a trypanosoma cruzi antibody and a preparation method of the kit. The kit comprises a component A and a component B, wherein the component A is a chagas antigen which is marked by a tracking marker or coated with a magnetic ball; the component B is a chagas second antibody which is coated with the magnetic ball or marked by the tracking marker; and any one of the component A and the component B is marked by the tracking marker and the other one is coated with the magnetic ball. The invention further provides a method for detecting the chagas antibody. By utilizing the kit provided by the invention, the concentration of chagas in a sample can be accurately and flexibly determined. On the basis, a pre-treatment solution is used for carrying out pre-treatment optimization so that the background interference can be reduced and the detection flexibility is improved. Meanwhile, detection of the full-automatic chemiluminiscence method can be realized with the help of a chemiluminiscence immunoassay analyzer, so that the operation time is shortened and manual operation errors are reduced.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
CSFV antibody detection system and preparation method thereof
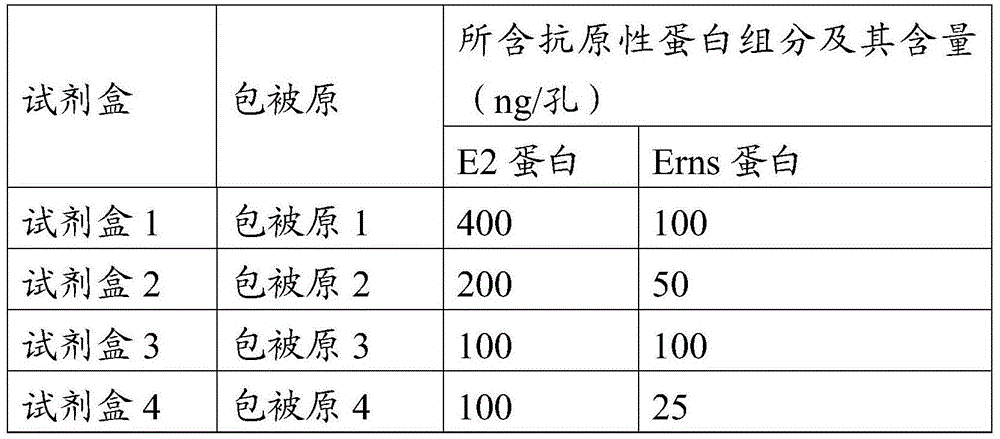
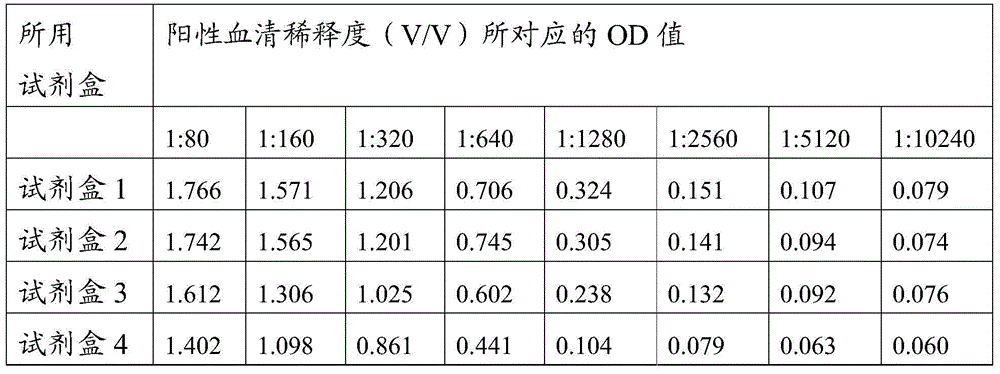
ActiveCN105527442AHigh detection rate sensitivityImprove capture efficiencyBiological testingSerum igeE2 protein
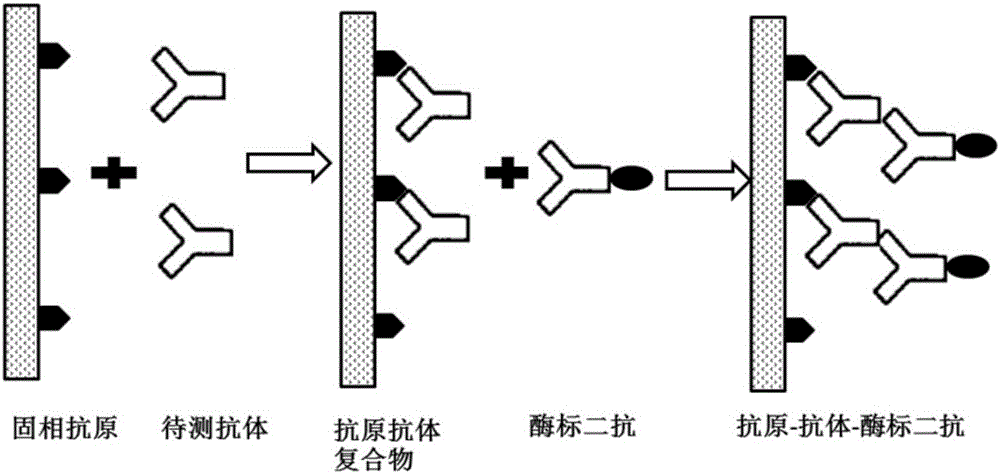
The invention provides a CSFV antibody detection system and a preparation method thereof. A coating antigen of the detection system contains CSFV E2 protein and Erns protein. The CSFV E2 protein and Erns protein are recombinant proteins expressed by eucaryon, correct spatial conformation and posttranslational modification process can be guaranteed, antigen is capable of effectively combining with the antibody in serum, and specificity, sensitivity and repeatability of detection can be increased. The system and the method can be sued for diagnosis of CSFV antibody in prevention and control of CSFV as well as immunization evaluation of a CSFV vaccine.
Owner:LUOYANG PULIKE WANTAI BIOTECH
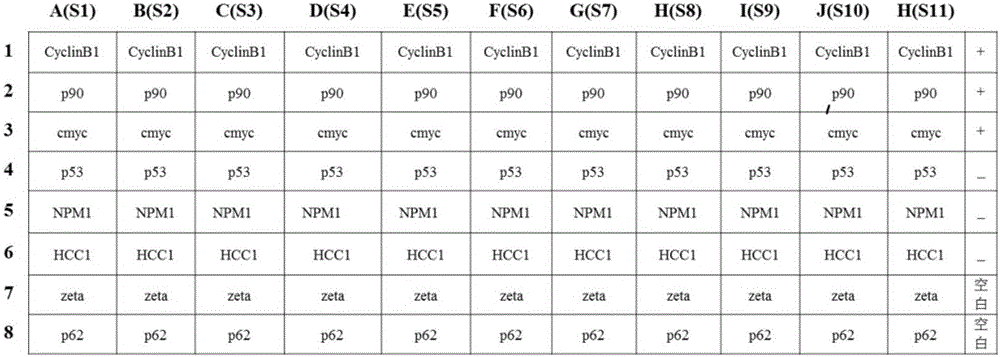
Multi-autoantibody joint detection ELISA kit for early screening and diagnosis on liver cancer
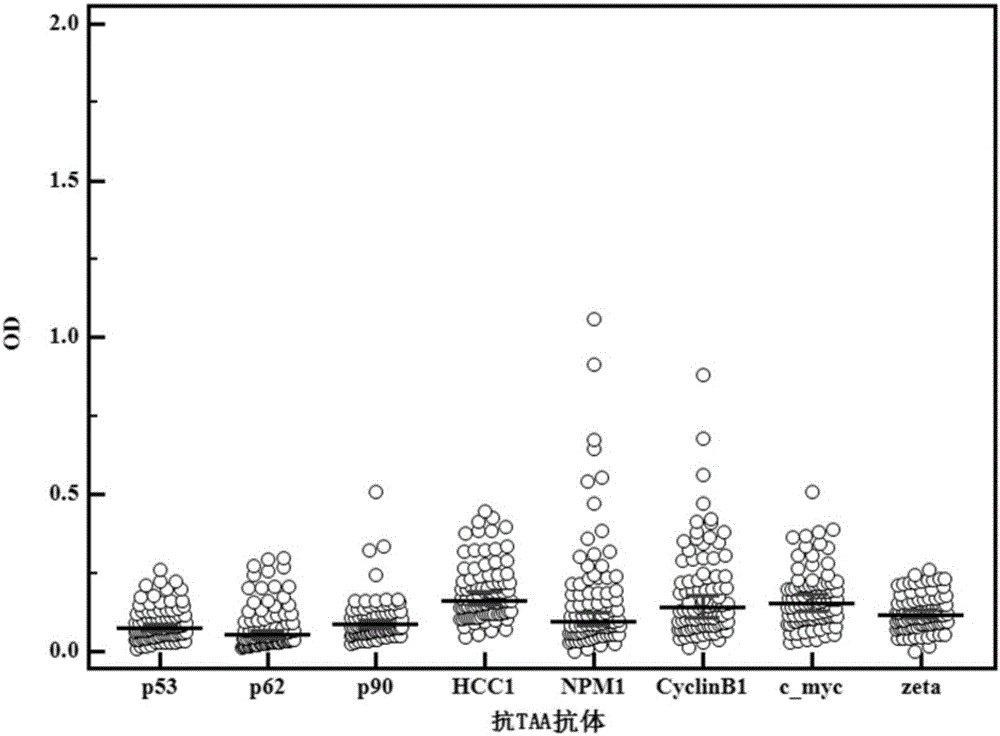
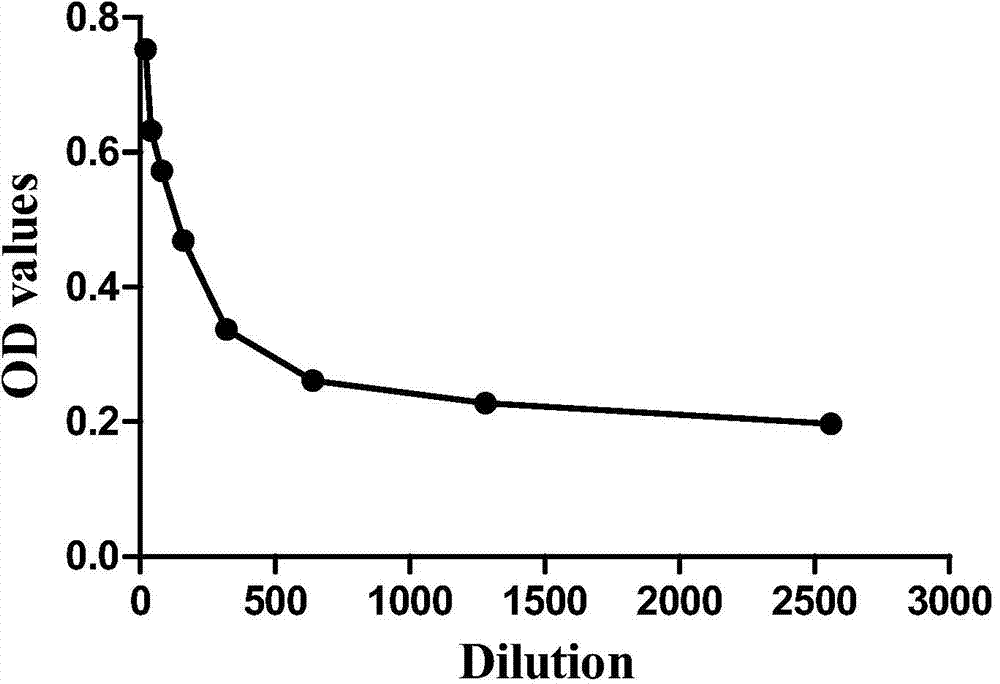
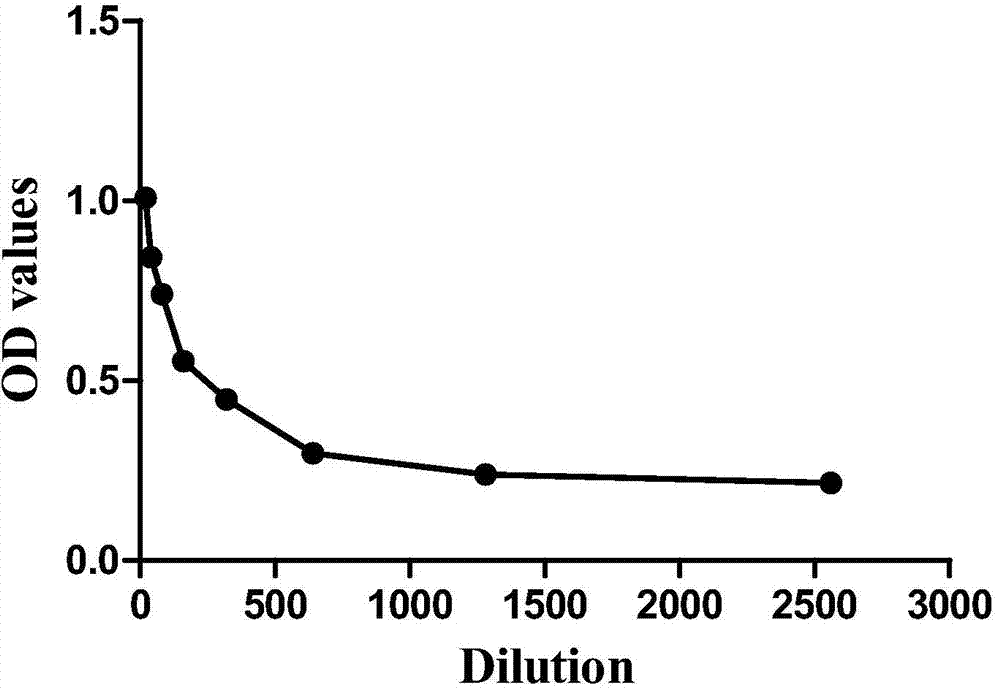
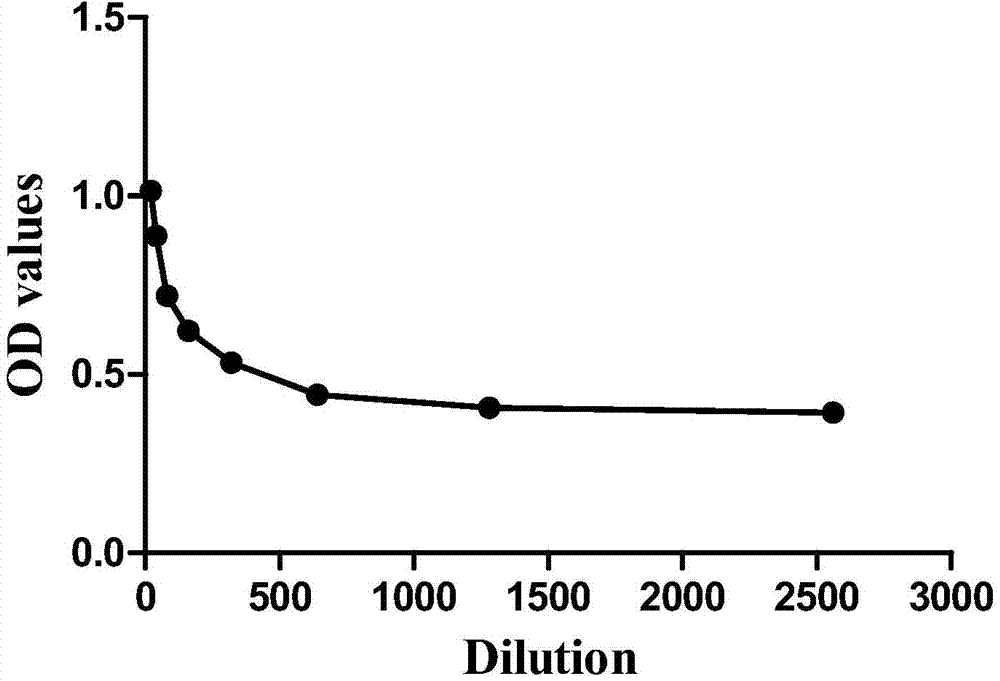
The invention discloses a multi-autoantibody joint detection ELISA kit for early screening and diagnosis on liver cancer. The kit contains a solid carrier and tumor-associated antigen, wherein the solid carrier is coated by the tumor-associated antigen; the tumor-associated antigen is CyclinB1, p90, c-myc, p53, NPM1, HCC1, 14-3-3zeta and p62. Furthermore, the kit further contains sample diluent, a second antibody, second antibody diluent, positive control serum, negative control serum, a developing liquid, a termination liquid and a washing liquid. The ELIS kit disclosed by the invention is applied to early screening on liver cancer and has the advantages of high sensitivity, good stability, simplicity and convenience in operation and the like. In addition, the ELISA kit disclosed by the invention is capable of detecting 11 blood samples simultaneously, so that the cost can be lowered, the efficiency can be improved, and patients suffering from liver cancer can be diagnosed and treated before occurrence of clinical symptoms.
Owner:厦门生迪生物技术有限公司
Kit for detecting protein glycosylation subtype and detection method thereof
InactiveCN104502611AWide applicabilityAchieve semi-quantitative detectionBiological testingBiotin-streptavidin complexPolystyrene
The invention discloses a kit for detecting protein glycosylation subtype and a detection method thereof. The detection kit comprises agglutinin, confining liquid, a biotin labeled antibody, horse radish peroxidase labeled streptavidin, a 3',3',5',5'-tetramethyl benzidine substrate developing solution, a stop solution and a cleaning buffer solution. The detection method comprises the following steps: fixing the agglutinin onto a polystyrene solid phase carrier to be used for recognizing and binding glycoprotein in the sample based on specific recognition of agglutinin and an oligosaccharide chain, recognizing the glycoprotein needing to be detected by virtue of specific binding of the protein and the antibody thereof, and realizing semi-quantitative analysis of the glycoprotein. According to the detection kit and the detection method disclosed by the invention, semi-quantitative detection of single protein glycosylation subtype can be realized, and the kit is easy and convenient to operate and low in cost.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
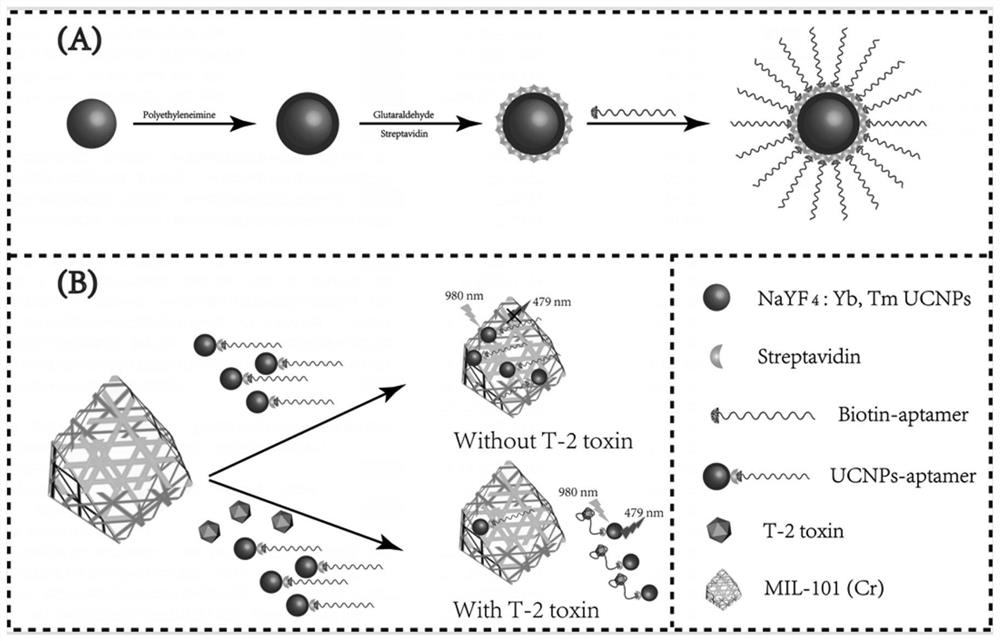
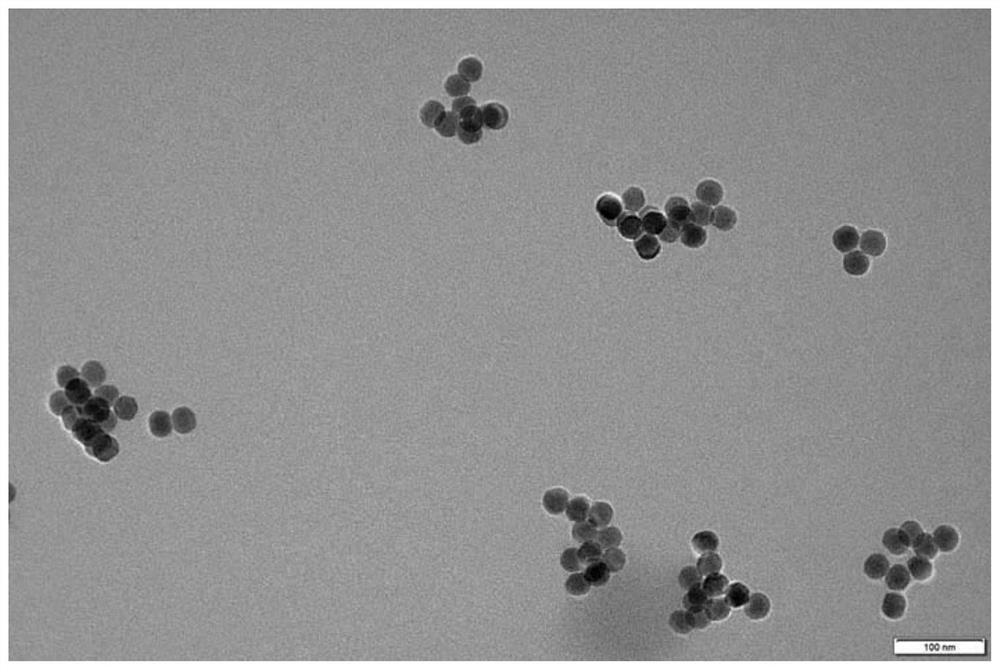
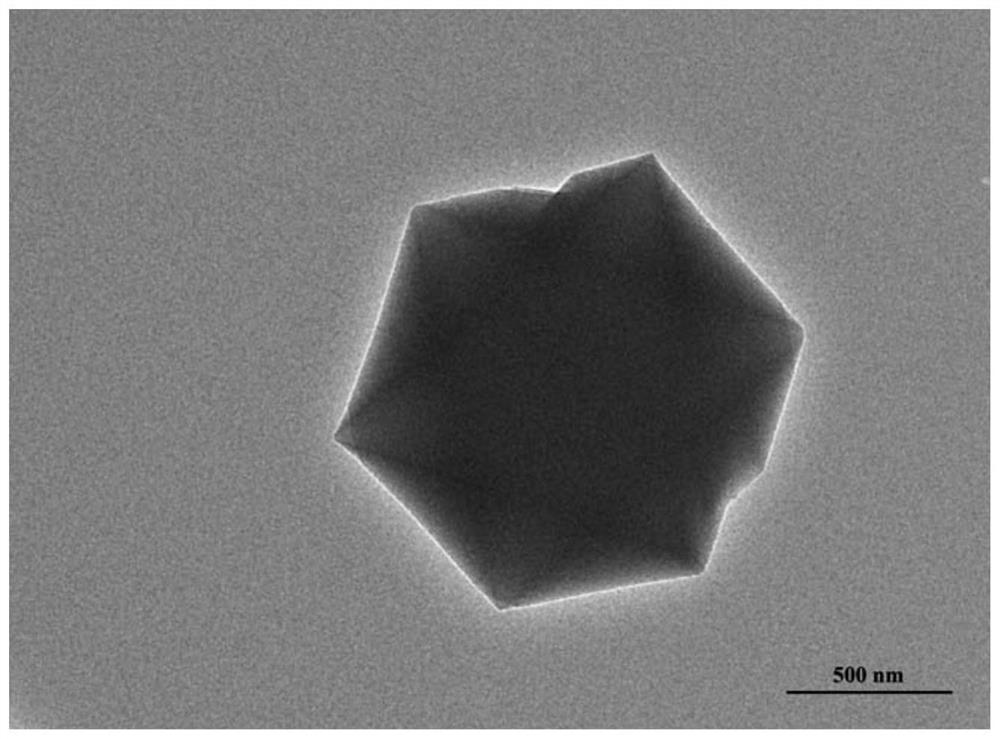
Method and kit for fluorescence detection of micromolecular mycotoxin based on metal organic framework and upconversion nanoparticles
ActiveCN112444510ARealize highly sensitive detectionOptimize the addition ratioFluorescence/phosphorescenceAptamerMetal-organic framework
The invention belongs to the field of micromolecular detection, and relates to a method and a kit for fluorescence detection of micromolecular mycotoxin based on a metal organic framework and upconversion nanoparticles. The method comprises the following steps: S1, obtaining an up-conversion nanoparticle probe modified with a micromolecular mycotoxin aptamer; S2, carrying out synthesis and activation of MIL-101 (Cr); S3, combining the micromolecular mycotoxin with the aptamer; and S4, carrying out signal detection: detecting a fluorescence signal of a product obtained by the reaction in the step S3 by adopting a fluorospectro photometer. The upconversion nanoparticles doped with rare earth are adopted, the fluorescence intensity is stable, the background value is low, and compared with other methods, the fluorescence detection kit does not need complex operation steps, is high in applicability, high in detection result sensitivity and good in specificity, and can be applied to rapid detection of on-site samples.
Owner:INST OF ENVIRONMENTAL MEDICINE & OCCUPATIONAL MEDICINE ACAD OF MILITARY MEDICINE ACAD OF MILITARY SCI
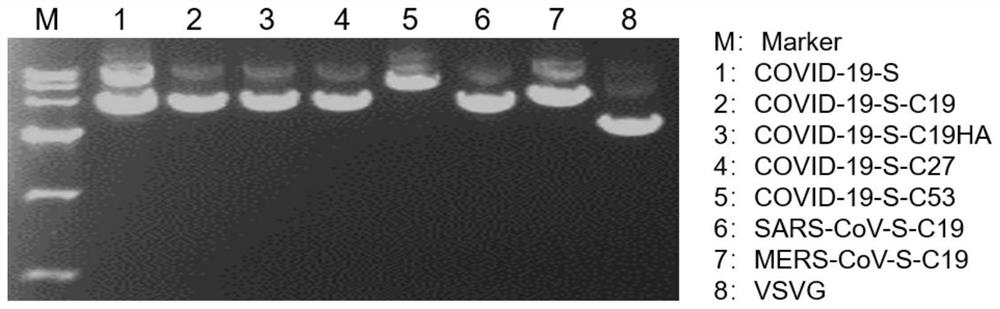
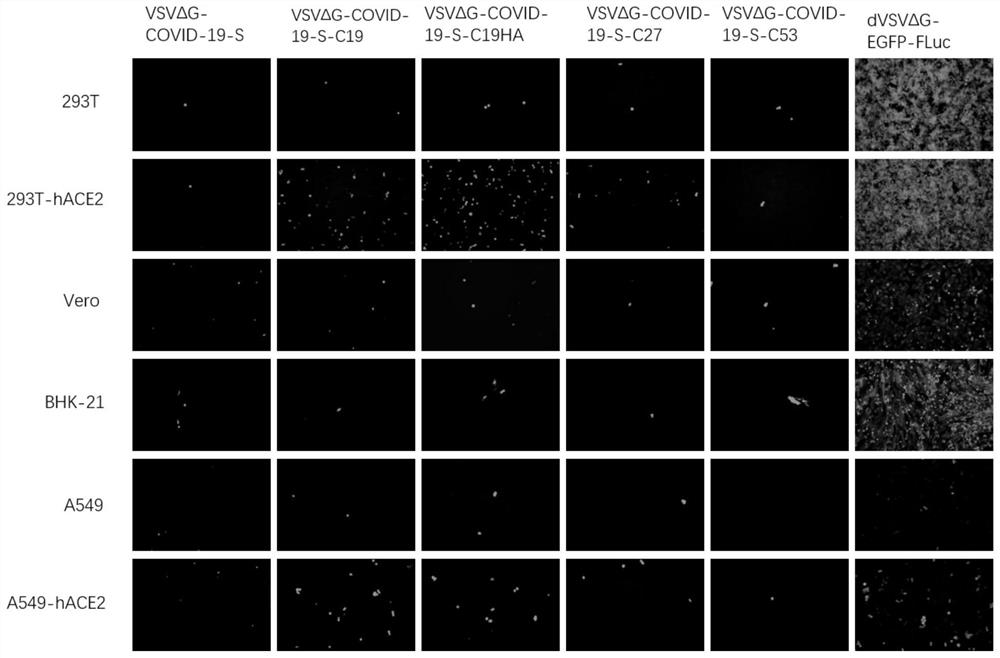
Coronavirus pseudovirus packaging system and packaging method, and application of coronavirus pseudovirus to evaluating disinfection efficacy
ActiveCN112760297AFast packLow background valueSsRNA viruses negative-senseBiocideDisinfectantFluorescent protein
The invention relates to a coronavirus pseudovirus packaging system. The coronavirus pseudovirus packaging system comprises a vesicular stomatitis virus VSV vector and an assembly cell, wherein the vesicular stomatitis virus VSV vector is formed by replacing GP genes with Fluc and EGFP double reporter genes, and the assembly cell is used for expressing coronavirus spike protein S. The double reporter genes are selected from luciferase and fluorescent protein, and the luciferase reporter gene is preferably the Fluc gene. According to the packaging system, a one-step packaging method is adopted, so that pseudoviruses which are infected in a single cycle, low in background value and high in titer and have the characteristic of rapid detection compared with a lentivirus-mediated pseudovirus system can be rapidly packaged, and the packaging system can be used for researching coronaviruses such as COVID-19 (SARS-CoV-2), SARS (SARS-CoV) and MERS; and the pseudoviruses can be used for evaluating the efficacy of a disinfectant through the steps of a virus pollution distribution model, scene building and sampling detection, a safe, convenient and effective tool method is provided for evaluating the disinfectant, and the pseudoviruses have wide application value.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
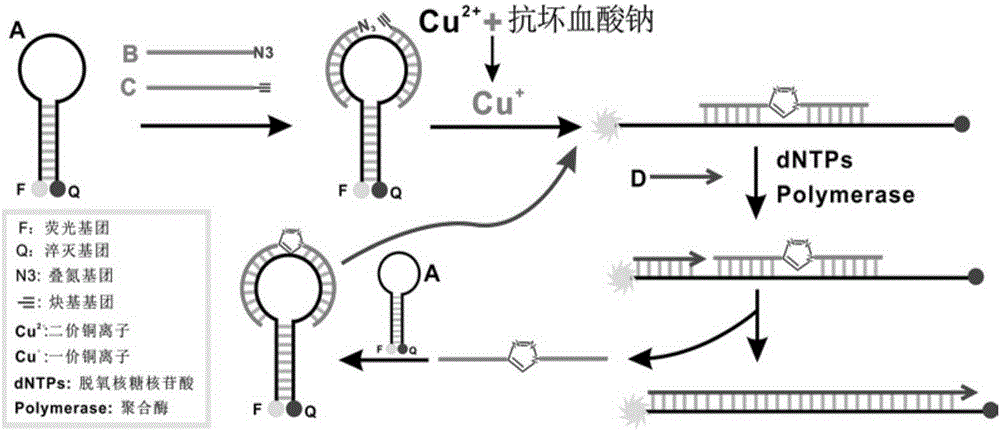
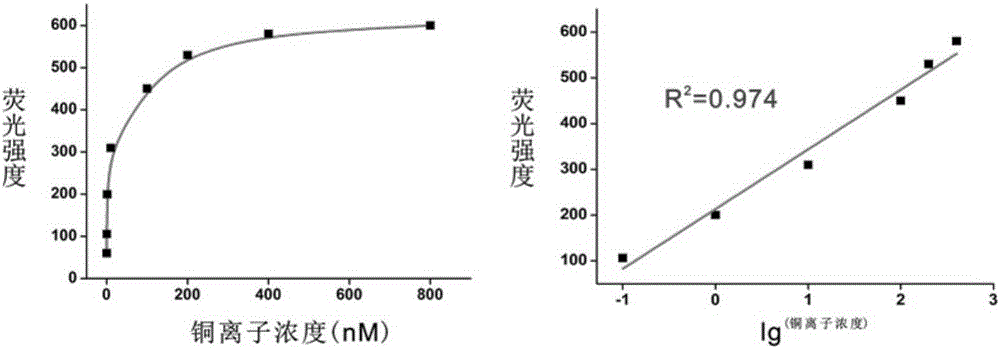
Copper ion detection method and detection kit thereof
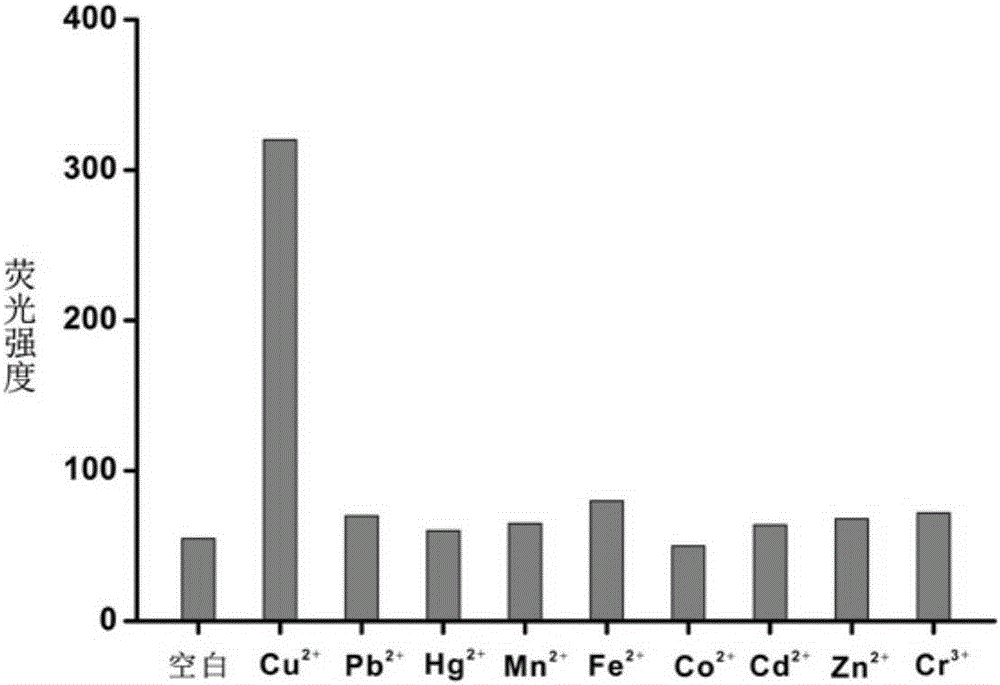
The invention discloses a copper ion detection method and a detection kit thereof. An azide group of a molecule and an alkynyl group of the other molecule are used, under catalysis of monovalent copper ions, a cycloaddition reaction is carried out to form triazole five-membered ring, and the azide group and the alkynyl group are connected, so that a hairpin structure for opening a fluorescence probe by a nucleic acid sequence is initiated, copper ions detection can be realized through amplification of an isothermal signal, and the detection kit has the advantages of good stability, high sensitivity, good selectivity, strong anti-interference capability, low cost and no toxicity, and strong practicality, and is especially suitable for detection and analysis of an environmental sample.
Owner:GUANGDONG INST OF ECO ENVIRONMENT & SOIL SCI
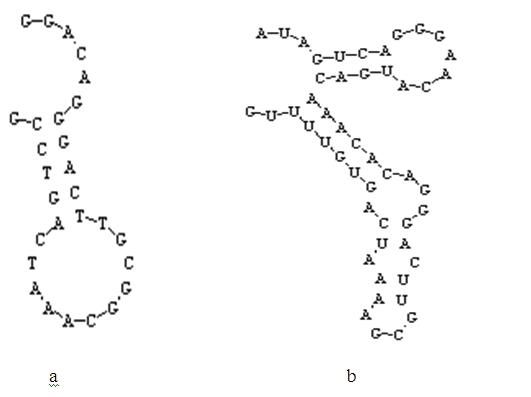
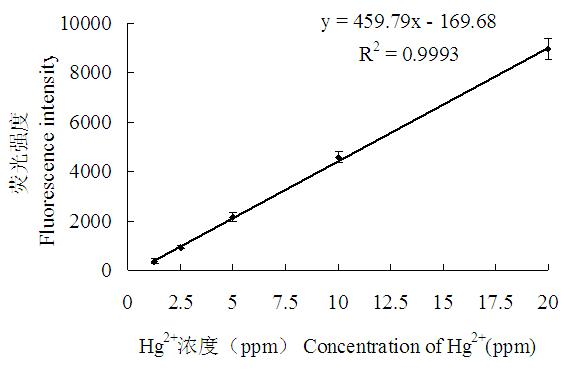
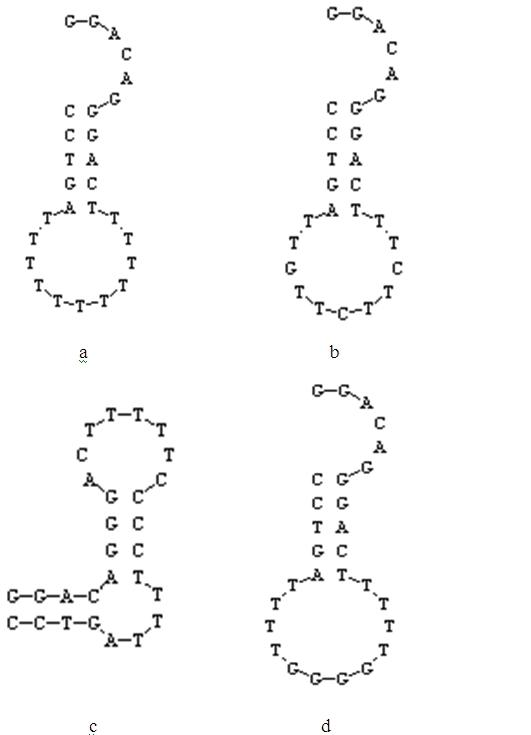
Method for detecting mercury ion residue of fluorescent signal conversion mechanism based on nucleic acid aptamer structure
InactiveCN102621120AHigh binding activityGood structure recognition functionFluorescence/phosphorescenceAptamerCompetitive binding
The invention relates to a method for detecting mercury ion residue of a fluorescent signal conversion mechanism based on a nucleic acid aptamer structure. In the method, a mercury ion nucleic acid aptamer marked with a fluorescein molecule, and a complementary sequence marked with a quenching element are utilized to form a fluorescent detection system, wherein the mercury ion nucleic acid aptamer is a stem loop structure composed of 27-28 bases and owns a stem formed by covalent binding of the bases GGAC and GTCC; and the base sequence of the complementary sequence Q2 is shown in SEQ ID NO.1. The method for detecting the mercury ion residue by the fluorescent detection system comprises the following steps of: adding mercury ions with a serial concentration and a complementary sequence Q2 competitive binding nucleic acid aptamer; establishing a standard curve according to the change of fluorescent signals and determining the lowest detection limit and linear range; and then, carrying out marking detection on a sample to be detected and judging the content of the mercury ions in the sample according to the standard curve. The method disclosed by the invention has the advantages of rapidness, simplicity, high sensitivity and selectivity and less sample quantity demand, and is applicable to the detection of the mercury ions in the actual sample.
Owner:JIANGSU ACAD OF AGRI SCI
Novel print recognition based metronidazole electrochemical sensor, preparation method and application
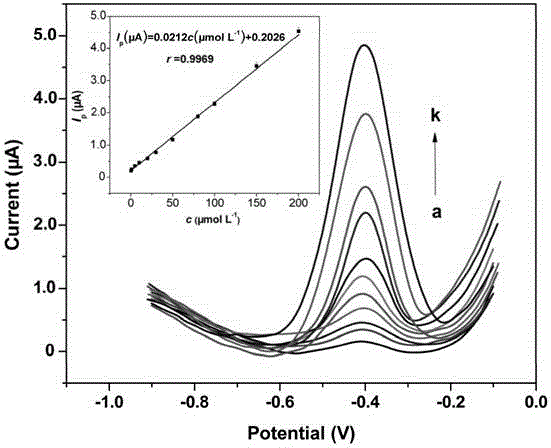
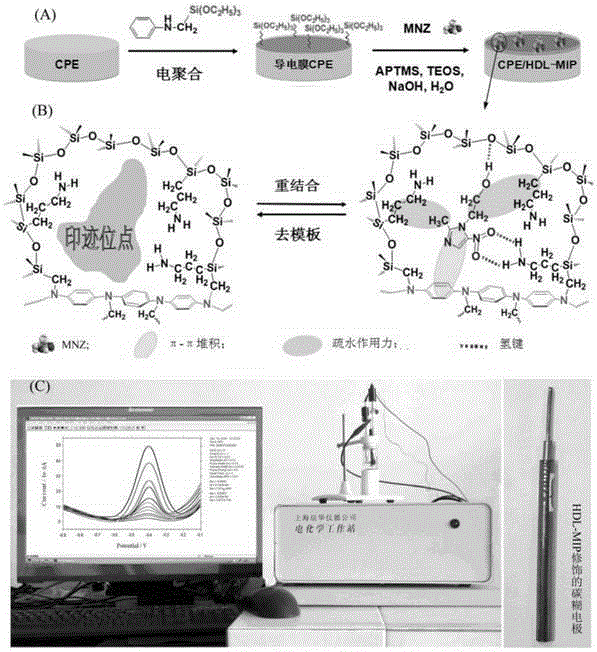
InactiveCN105181780AWide applicabilityDirect Efficient Signal ConversionMaterial electrochemical variablesModified carbonMolecularly imprinted polymer
The invention provides a novel print recognition based metronidazole electrochemical sensor, a preparation method and an application. The preparation method of the sensor comprises following steps: graphite powder and paraffin oil are uniformly mixed to form carbon paste, a carbon paste electrode is prepared and put in a sulfuric acid solution for potential scan round, and a conductive film carbon paste electrode is prepared; sol proportionally prepared from APTMS (aminopropyl trimethoxysilane), TEOS (tetraethoxysilane), 2-ethoxyethanol, a metronidazole containing 2-ethoxyethanol solution, an NaOH solution and deionized water is dispensed on the surface of the conductive film carbon paste electrode dropwise, a heterogeneous double-layered-based molecularly imprinted polymer film modified carbon paste electrode is obtained and connected with an electrochemical device, and the sensor is assembled. The sensor has enhanced print recognition capacity and wide applicability, has the characteristics of high sensitivity, good selectivity, wide linear range, high precision and accuracy, low detection cost, environment-friendliness, portability, suitability for field monitoring and the like, further, the preparation process is simple, the cost is low, and the service life is long.
Owner:NANHUA UNIV
Dry-type immunofluorescence kit for detecting Alzheimer syndrome MS4A6A and preparation method thereof
InactiveCN105759057AStrong specificityImprove accuracyDisease diagnosisBiological testingImmunofluorescenceNitrocellulose
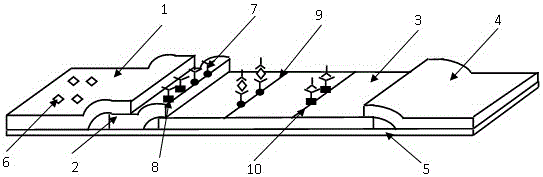
The invention relates to a dry-type immunofluorescence kit for detecting Alzheimer syndrome MS4A6A and a preparation method thereof. The kit comprises a PVC (polyvinyl chloride) bottom plate, a fluorescent-binding pad, a nitrocellulose membrane, a sample pad and a water absorbent pad, wherein the sample pad, the nitrocellulose membrane, the fluorescent-binding pad and the water absorbent pad are fixed on the PVC bottom plate horizontally; a coated mouse anti-human MS4A6A monoclonal antibody 1-nano-fluorescence bead compound and a coated chicken IgY-nano-fluorescence bead compound are arranged on the fluorescent-binding pad, and a detection line formed by mouse anti-human MS4A6A monoclonal antibody 2 and a quality control line formed by rabbit anti-chicken IgY antibody are formed on the nitrocellulose membrane. According to the kit, the nano-fluorescence bead is used for detecting MS4A6A, the background value is low, the detection time is short, the specificity and the sensitivity are high, the detection result is accurate, and the kit and the method provide a more reliable basis for clinical diagnosis and Alzheimer syndrome.
Owner:上海凯璟生物科技股份有限公司
African swine fever virus antibody ELISA detection kit and preparation method thereof
ActiveCN111929433AImprove the detection rateHigh sensitivityMaterial analysisClassical swine fever virus CSFVAfrican swine fever virus Antibody
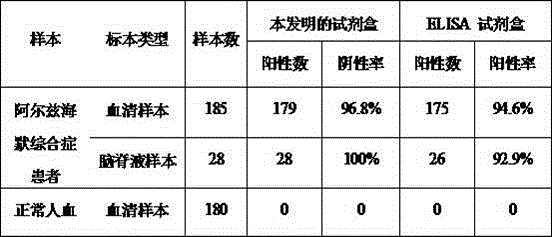
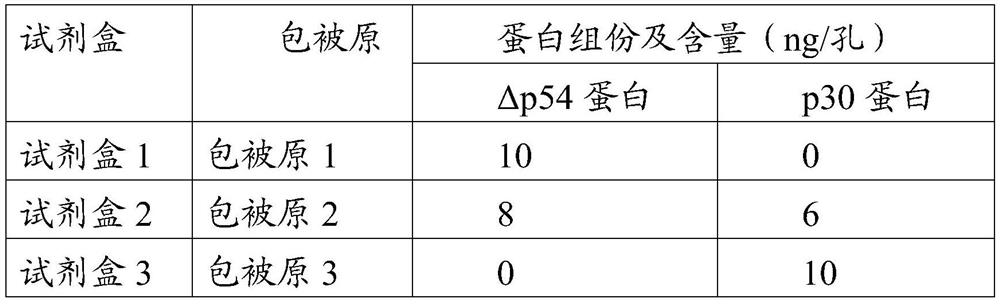
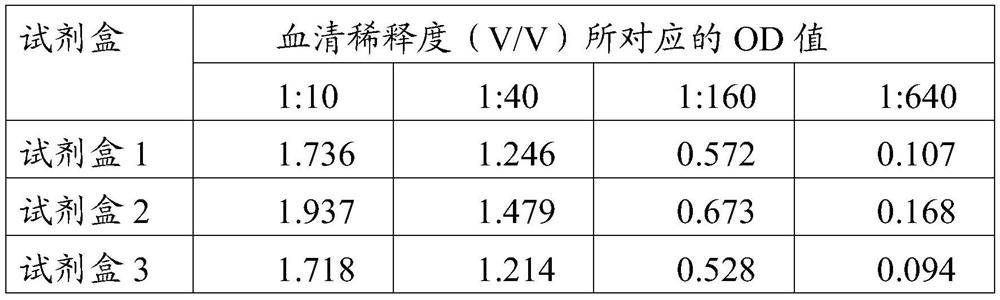
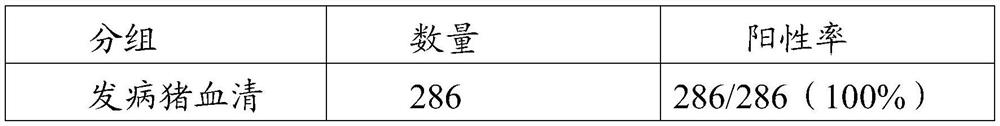
The invention provides an African swine fever virus antibody ELISA (Enzyme Linked Immuno Sorbent Assay) detection kit. A support medium of the kit is coated with an African swine fever virus antigen,and the African swine fever virus antigen is African swine fever virus p30 and / or protein delta p54 protein. The African swine fever virus p30 protein is as shown in SEQ ID No. 1, and the African swine fever virus delta p54 protein is as shown in SEQ ID No. 2. The African swine fever virus antibody ELISA detection kit disclosed by the invention is good in specificity, sensitivity and repeatability, the sensitivity of the kit coated with the two antigens is equivalent to that of indirect immunofluorescence detection, antibody positive can be detected earlier at a low antibody level in the earlystage, a basis is provided for clinical swine herd infection conditions, and the kit plays an important role in preventing and controlling African swine fever virus infection.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Electrode modification method for adopting nucleic acid aptamers for high sensitivity detection of tumor cells
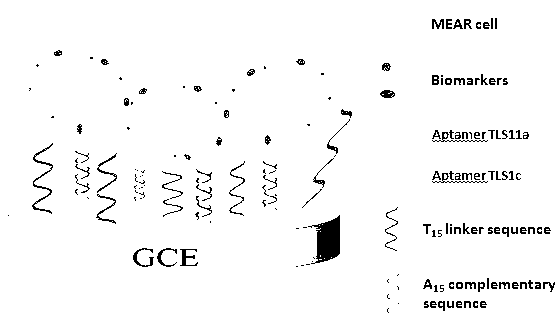
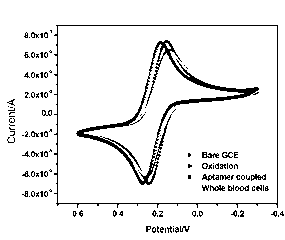
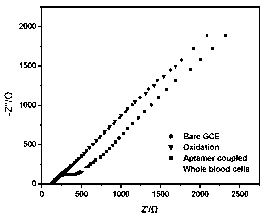
InactiveCN103257167AHigh sensitivityLow background valueMaterial analysis by electric/magnetic meansAptamerDifferential pulse voltammetry
The invention discloses an electrode modification method for adopting nucleic acid aptamers for high sensitivity detection of tumor cells, wherein hepatic carcinoma cell BNL1MEA.7R.1(MEAR)-specific aptamers TLS1c and TLS11a are covalently modified on the surface of an electrode, and a cyclic voltammetry method, a differential pulse voltammetry method and an alternating current impedance method are adopted to detect MEAR cells, such that trace detection on the single MEAR cell can be achieved in 1 mL of 1*10<9> blood cells. With the technical scheme, a background value and a detection limitation can be significantly reduced, and sensitivity of electrochemical tumor cell detection can be improved.
Owner:SUZHOU UNIV
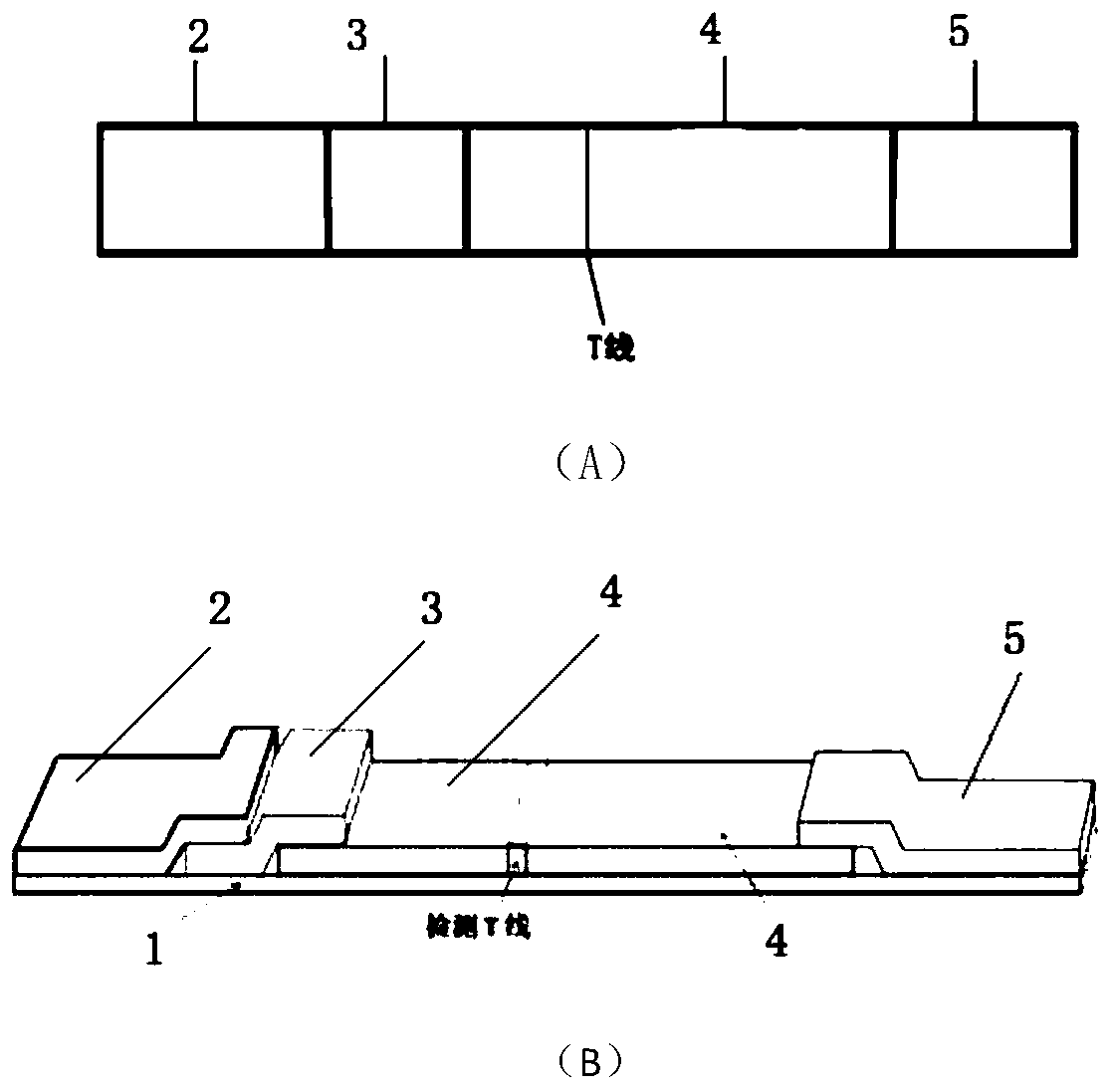
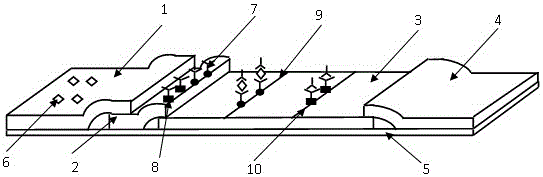
Fluorescence-quenched colloidal gold immunochromatographic test strip, preparation method and application of fluorescence-quenched colloidal gold immunochromatographic test strip
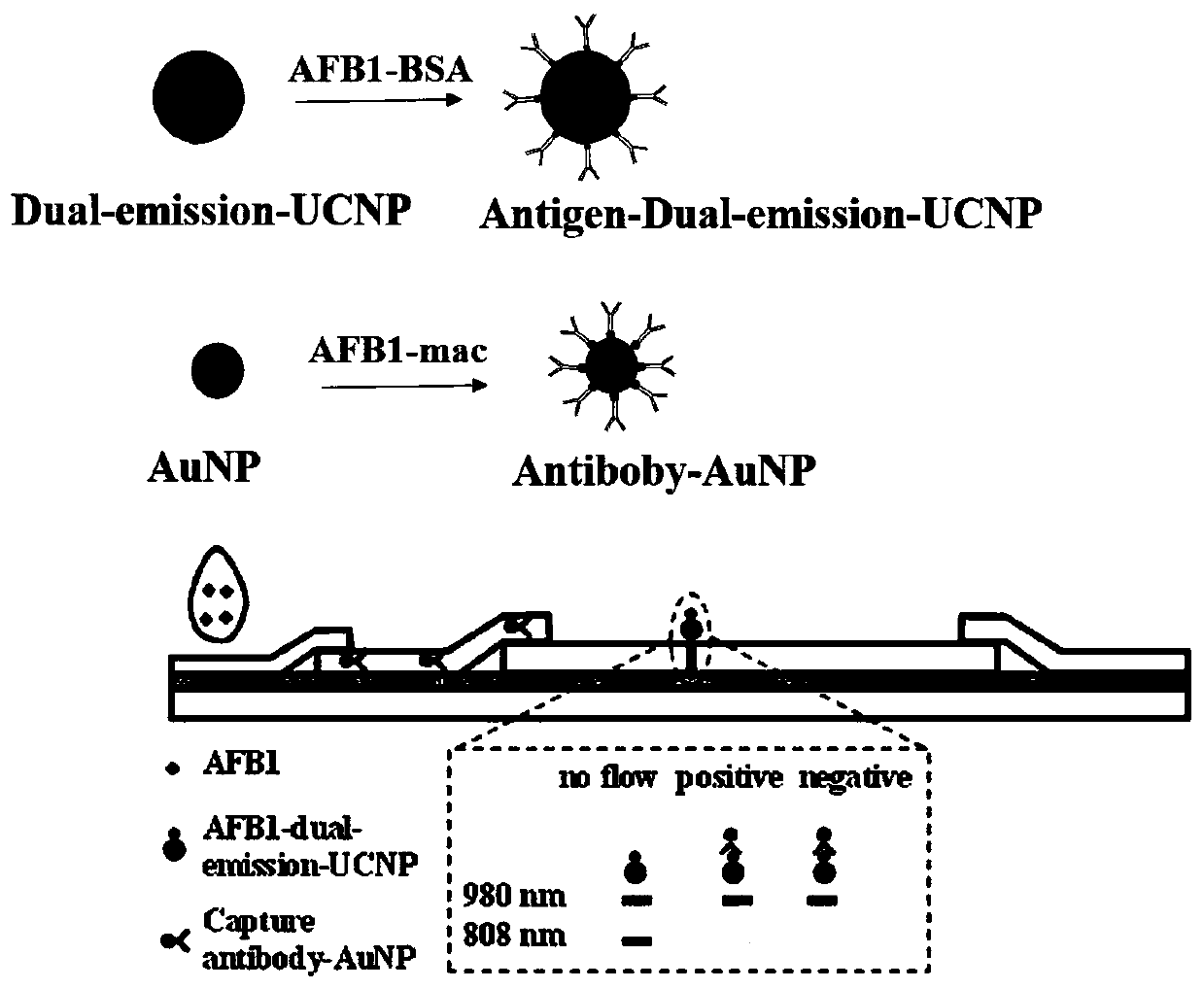
The invention provides a fluorescence-quenched colloidal gold chromatography test strip which comprises a bottom plate, and a sample pad, a combination pad, a nitrocellulose membrane and absorbent paper which are sequentially arranged on the bottom plate from left to right; the sample pad and the combination pad, the combination pad and the nitrocellulose membrane, and the nitrocellulose membraneand the absorbent paper are partially laminated; gold nanoparticles are arranged on the combination pad, and antibodies of a to-be-detected object are marked on the gold nanoparticles; an antigen anda detection T line of a dual-excitation dual-emission up-conversion nanoparticle labeled detection object are fixed on the nitrocellulose membrane; the up-conversion nanoparticles can emit red light or green light under the excitation of two different near-infrared lights at the same time. The invention also discloses a preparation method and a detection method of the kit. According to the chromatographic test strip and the detection method thereof provided by the invention, the result can be directly observed under the excitation of near-infrared light, the sensitive components are accuratelyand quantitatively detected, the background interference is eliminated, the background value is low, the signal is stable, and the sensitivity is greatly improved.
Owner:SHANGHAI UNIV
Method and apparatus for an integrity test of a test container
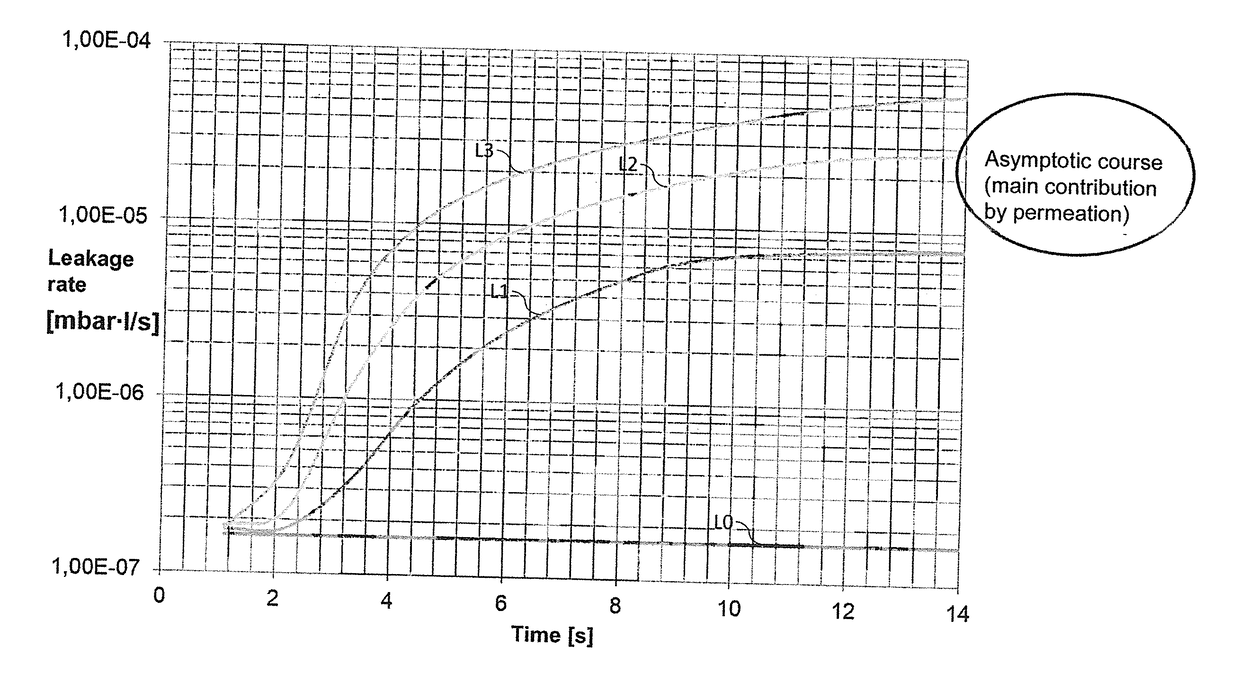
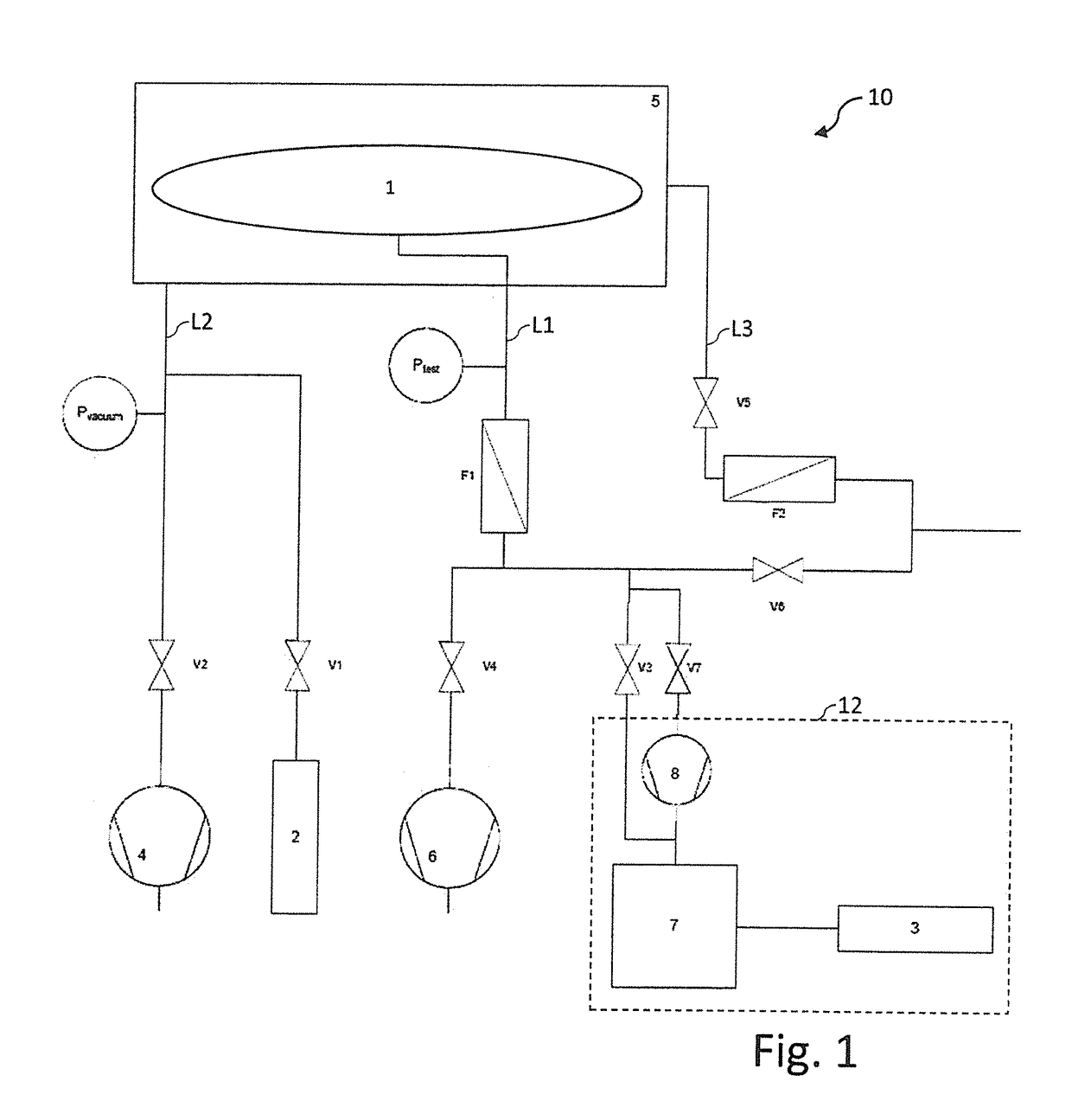
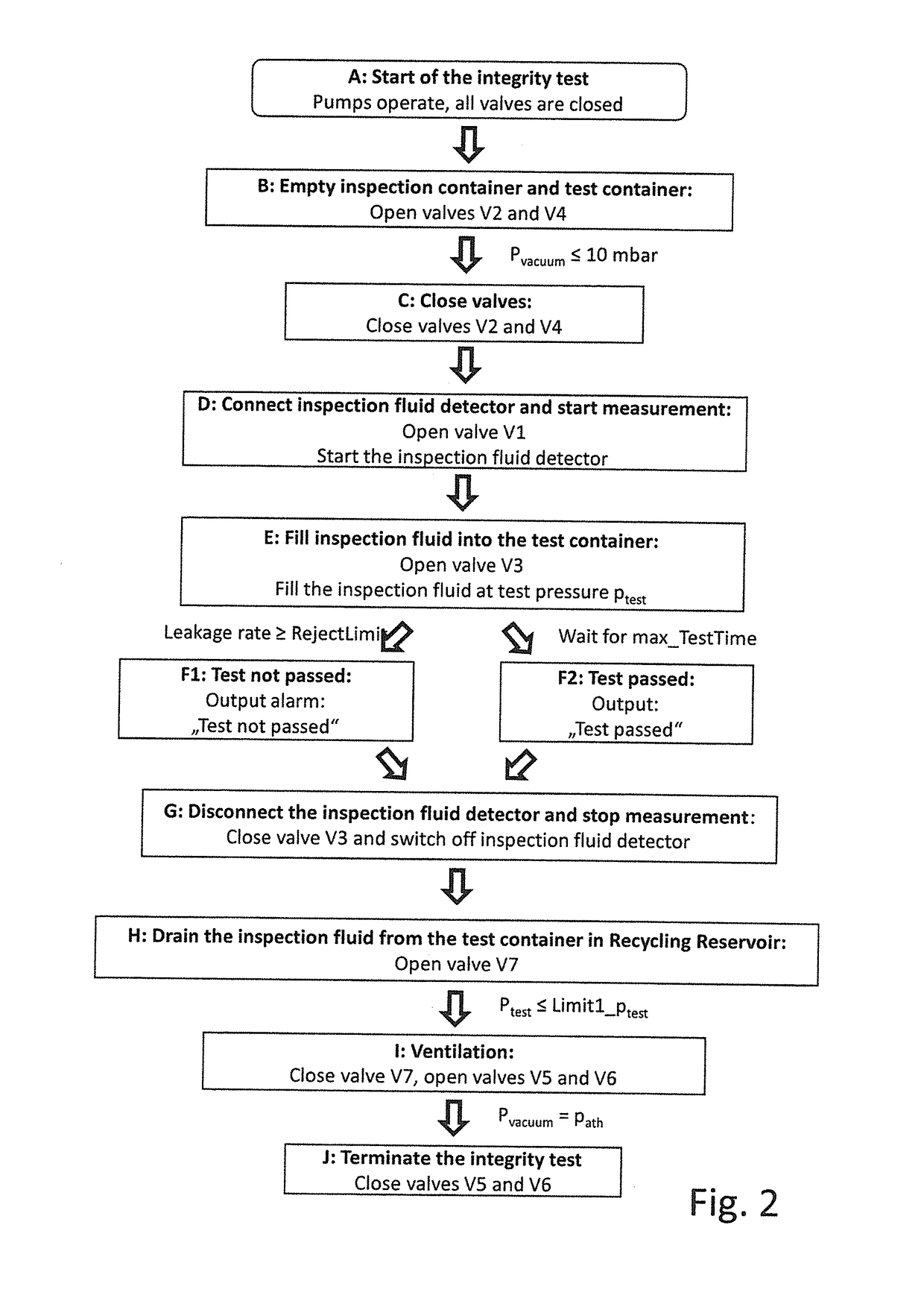
ActiveUS20180188130A1More timeReliable methodDetection of fluid at leakage pointMeasurement of fluid loss/gain rateEngineeringIntegrity testing
A method, a device and a use of the device are provided for carrying out an integrity test on a test container (1) that has at least one flexible casing material. The method includes the steps of: filling a test fluid into the test container (1); and detecting the presence of test fluid directly outside the test container (1). The test fluid is designed in such a way that the permeation rate of test fluid through undamaged casing material of the test container (1) is not greater than 1·10−6 mbar·m / (s·bar).
Owner:SARTORIUS STEDIM BIOTECH GMBH
Hepatitis C antibody polymer enzyme marker, and preparation and application thereof
ActiveCN107037207ALong-term effective preservationHigh sensitivityMaterial analysisCross-linkHydrazine compound
The invention discloses a hepatitis C antibody polymer enzyme marker. The Hepatitis C antibody polymer enzyme marker is prepared according to the following steps: with glucosan T500 as a polymer carrier, activating with hydrazine hydrochloride and then crosslinking with HRP, thereby acquiring polymer enzyme marker (Poly-HRP); modifying Hepatitis C antibody with sulfydryl and then cross-linking the Hepatitis C antibody modified with sulfydryl with the polymer enzyme marker (Poly-HRP). The invention also discloses a polymer enzyme stabilizer and a diluent which are used together with the hepatitis C antibody polymer enzyme marker. A test proves that the hepatitis C antibody polymer enzyme marker disclosed by the invention can be stably stored for two years after the polymer enzyme stabilizer is added, and meanwhile, after the hepatitis C antibody polymer enzyme marker is diluted with the diluent, the sensitivity of the diluted hepatitis C antibody polymer enzyme marker used for enzyme-linked immunosorbent assay and chemiluminescence immunodetection is greatly higher than that of a enzyme marker for a traditional sodium periodate method or glutaraldehyde method.
Owner:山东莱博生物科技有限公司
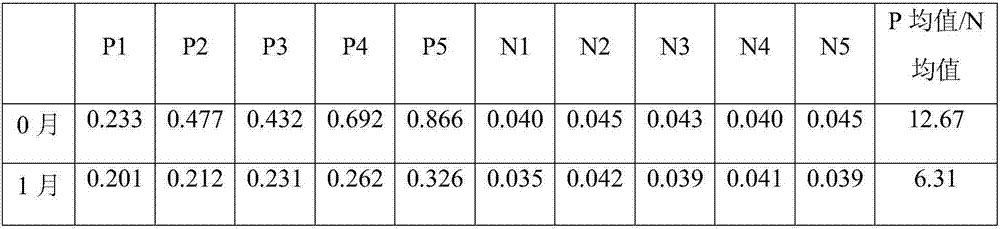
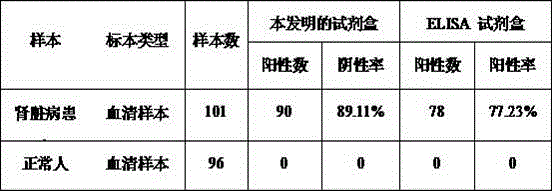
Chronic kidney disease marker suPAR detection kit and preparation method thereof
InactiveCN106556703AAccurate measurementImprove accuracyDisease diagnosisBiological testingNitrocelluloseMedicine
The invention relates to a chronic kidney disease marker suPAR detection kit and a preparation method thereof. The kit includes a PVC base board, a fluorescent combining pad, a nitrocellulose membrane, a sample pad, and a water absorption pad. The sample pad, the nitrocellulose membrane, the fluorescent combining pad and the water absorption pad are fixed on the PVC base board in the horizontal direction. A coated rat-anti-human suPAR monoclonal antibody 1-nano fluorescent microsphere compound and a chicken IgY-nano fluorescent microsphere compound are formed on the fluorescent combining pad; and a detection line, which is formed by an rat-anti-human suPAR monoclonal antibody 2, and a quality control line, which is fomed by a rabbit-anti-chicken IgY antibody, are formed on the nitrocellulose membrane. Through nano fluorescent microspheres for detection of suPAR, the kit has low background value, short detection time, high specificity and sensitivity and accurate detection result. The kit provides a more reliable basis for early stage clinical diagnosis and general survey of the chronic kidney disease.
Owner:上海凯璟生物科技股份有限公司
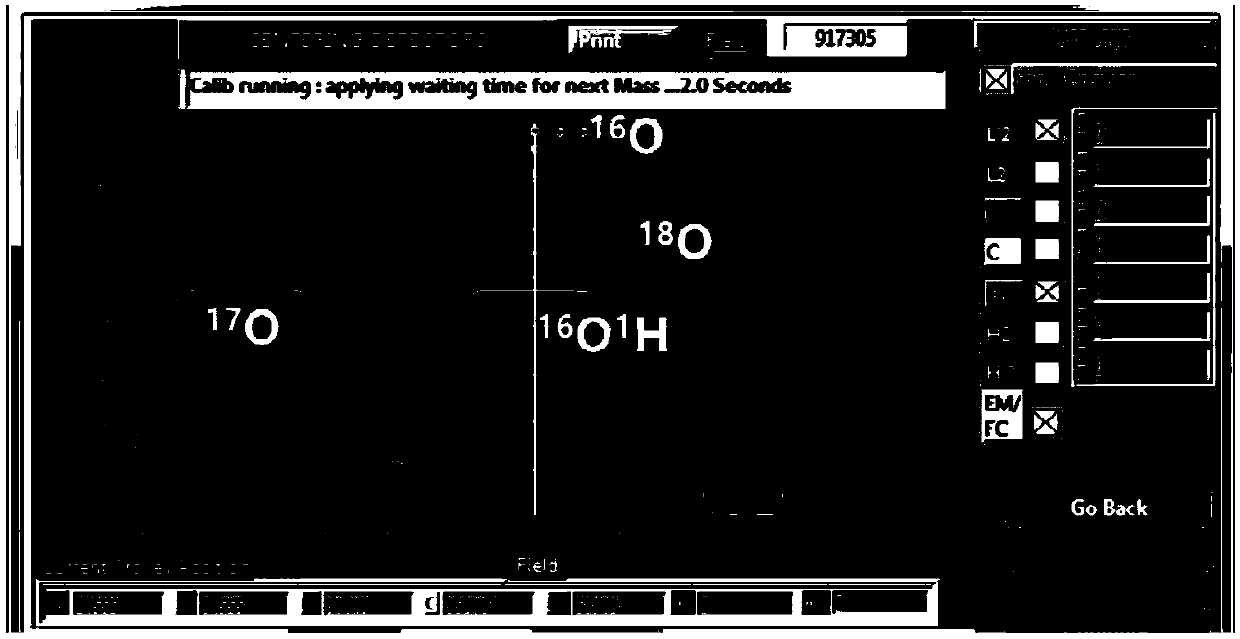
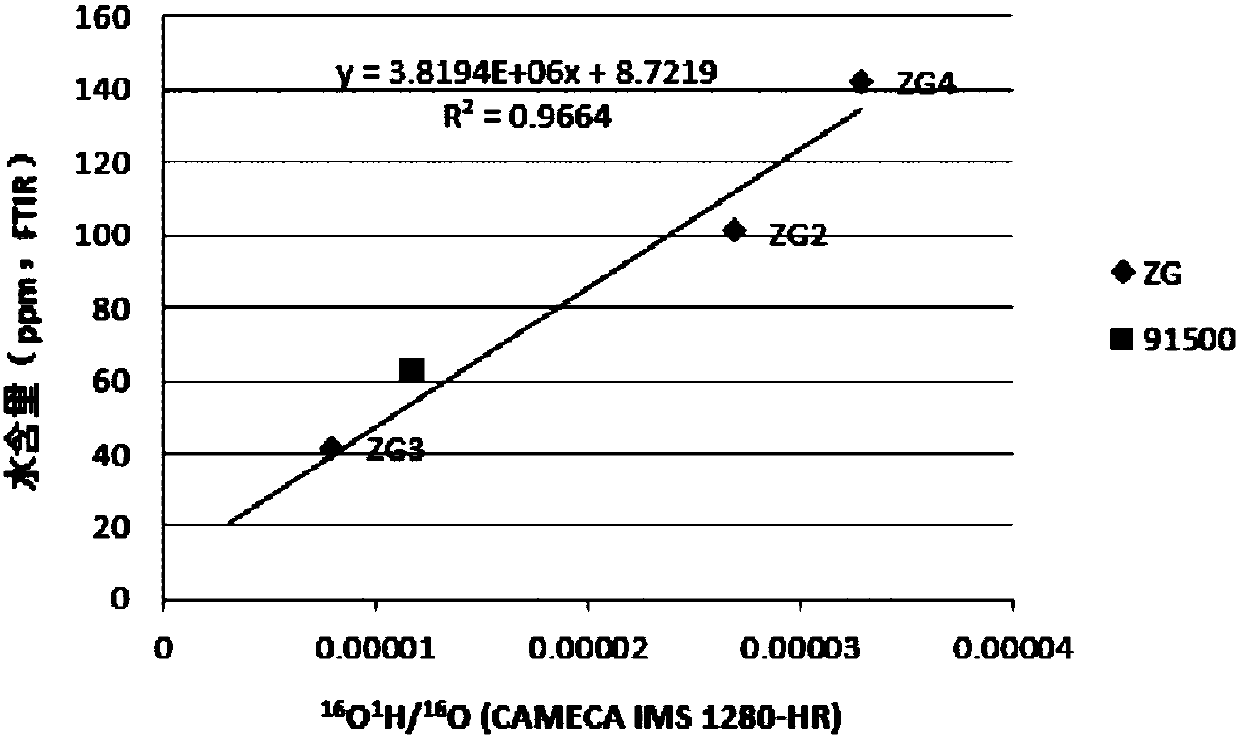
A method of simultaneously analyzing the 'water' content and oxygen isotope in zircon based on large-scale secondary ion mass spectrometry
ActiveCN108037172AIncrease vacuumLow detection limitPreparing sample for investigationMaterial analysis by electric/magnetic meansMass spectrum analysisImage resolution
A method of simultaneously analyzing the 'water' content and oxygen isotope in zircon based on large-scale secondary ion mass spectrometry is disclosed. The method is based on baking in an instrumentsystem and baking and air exhausting by a circulating titanium pump to reduce an internal background value of an instrument as far as possible, a target made of an alloy material is adopted to reduceimpurity gas introduced from the outside, and liquid nitrogen refrigeration is adopted as assist, and therefore the vacuum degree of a sample cavity is greatly increased, the lower limit of detectionis effectively reduced, and the analysis precision is increased. Based on a high spatial resolution characteristic of a large-scale secondary ion mass spectrometer, microcell in-situ simultaneous analysis of the 'water' content and the oxygen isotope for small zircon particles and other nominally anhydrous minerals is achieved.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Analysis method for detecting activity of beta-D-glucosidase in soil
PendingCN109557065AΒ-D-glucosidase activity exact testΒ-D-glucosidase activity exclusionFluorescence/phosphorescenceAlgluceraseAnalysis method
The invention discloses an analysis method for detecting activity of beta-D-glucosidase in soil, and belongs to the technical field of enzyme activity detection. According to the method, the enzyme activity is equal to [(RS-BU)*(SA-SC) / (QS-SC)]-(NC-BU)*B*2*300 / (0.3*W*200), wherein SC is a fluorescence intensity value measured by supernatant and 50 ul of acetic acid buffer solution, QS is a fluorescence intensity value measured by supernatant and 50 ul of master standard solution, SA is a fluorescence intensity value measured by supernatant and 50 uL of 4-methylumbelliferone-beta-D-glucosidasesubstrate solution, BU is a fluorescence intensity value measured by 200 ul of acetic acid buffer solution and 50 ul of acetic acid buffer solution, RS is a fluorescence intensity value measured by 200 ul of acetic acid buffer solution and 50 ul of standard substance solution, and NC is a fluorescence intensity value measured by 200 ul of acetic acid solution and 50 ul of 4-methylumbelliferone-beta-D-glucosidase substrate solution. The method is capable of decreasing the background values, eliminating the background values interacted by samples and fluorophores, eliminating the background values, for standard substances, of buffer solution, improving the correctness of measurement results, and making the sample and drug dosages less.
Owner:JILIN ACAD OF AGRI SCI
Enzyme linked immunosorbent assay kit for quantitatively detecting CD79 alpha
ActiveCN106153935AOvercome the defects that are not suitable as ELISA reagentsAdd binding sitesBiological testingBiotin-streptavidin complexElisa kit
The invention relates to an enzyme linked immunosorbent assay kit for quantitatively detecting CD79 alpha. The enzyme linked immunosorbent assay kit for quantitatively detecting CD79 alpha comprises an enzyme-labeled panel coated with a joint capture antibody, confining liquid, washing liquid, a CD79 alpha standard product solution, substrate developing liquid, stopping liquid, an anti-CD79 alpha monoclonal antibody detection antibody and streptavidin labeled by pepper peroxidase. The joint capture antibody comprises an anti-CD79 alpha polyclonal antibody and an anti-CD79 alpha monoclonal antibody obtained by a fusion protein immunological experiment animal. The enzyme linked immunosorbent assay kit is easy to operate, short in time, high in sensitivity and suitable for rapid detection of a large number of samples.
Owner:广州瀚普创展医学检验实验室有限公司
Magnetic micro-particle chemiluminescence kit for determining content of interleukin-8 in human serum
PendingCN113804896ALow pre-processing requirementsNo pollution in the processChemiluminescene/bioluminescenceBiological material analysisAntigenMonoclonal antibody agent
The invention relates to a magnetic micro-particle chemiluminescence kit for determining the content of interleukin-8 in human serum. The magnetic micro-particle chemiluminescence kit comprises a reagent R1, a reagent R2, a magnetic separation reagent, a calibration product and a quality control product, wherein the reagent R1 is prepared from a biotinylated IL-8 monoclonal antibody A and a reagent R1 diluent, the reagent R2 is prepared from an alkaline phosphatase labeled IL-8 monoclonal antibody B and a reagent R2 diluent, the magnetic separation reagent is prepared from commercial streptavidin magnetic beads and a magnetic separation reagent diluent, the calibration product and the quality control product are prepared from diluents containing IL-8 antigens with different concentrations and proteins, and a chemiluminescent substrate solution required in an experiment process is an alkaline phosphatase catalytic luminescence substrate solution. According to the invention, a chemiluminescence detection technology and a biotin-streptavidin system are combined for the first time to realize the purpose of quantitative detection of the content of IL-8 in a human serum sample, and a more accurate, precise, convenient, rapid and simple method is provided for clinical detection of IL-8 in human serum.
Owner:BEIJING LEADMAN BIOCHEM
Up-conversion luminescence-based chip detection system and reagent for gynecological tumor markers CA125, CA153, SCCA, HE4
The invention relates to an up-conversion luminescence-based detection system for four gynecological tumor markers (CA125, CA153, SCCA, HE4), and the detection is performed by using a reaction cup detection system. The reaction cup detection system comprises a carrier plate, a reaction detection cup, and a fluorescence detector. The reaction detection cup is composed of a reaction detection cup and a test tube cover. A sample is dropwise added into the reaction detection cup for detection; after reaction for 5-15 min, the carrier plate is taken out and inserted into a slot of the fluorescence detector for value reading. The invention is characterized in that: the detection of four gynecological tumor markers CA125, CA153, SCCA, HE4 is carried out simultaneously by combining with an up-conversion luminescence detection method. The background value is small, and the measurement is rapid, accurate, and simple in operation. Data are transmitted to a doctor through wifi so as to obtain the detection results rapidly, and thus the purpose of rapid diagnosis is realized.
Owner:上海凯璟生物科技有限公司
Analyses testing method of aluminum, calcium, iron, molybdenum, niobium, titanium, tungsten impurity elements in chromium carbide
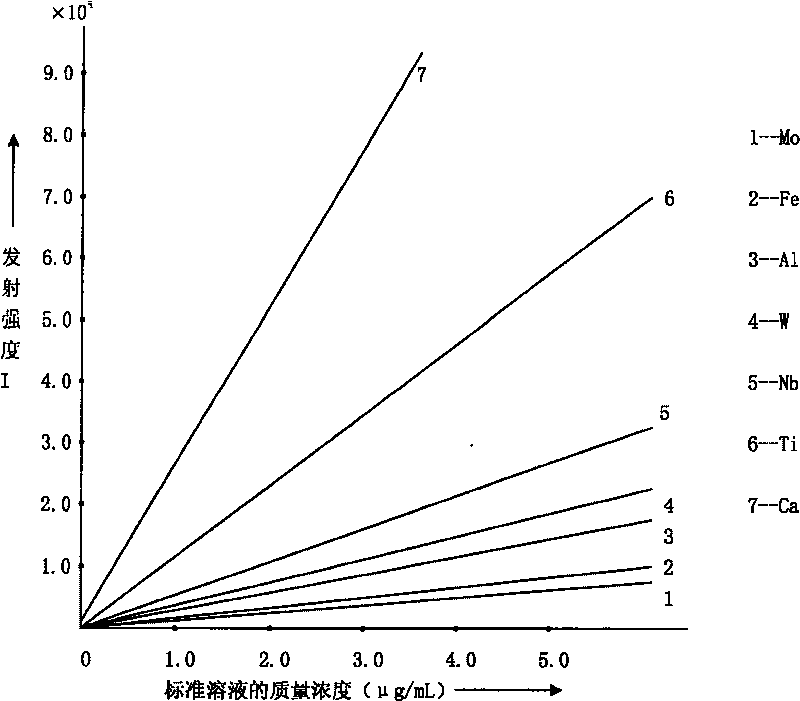
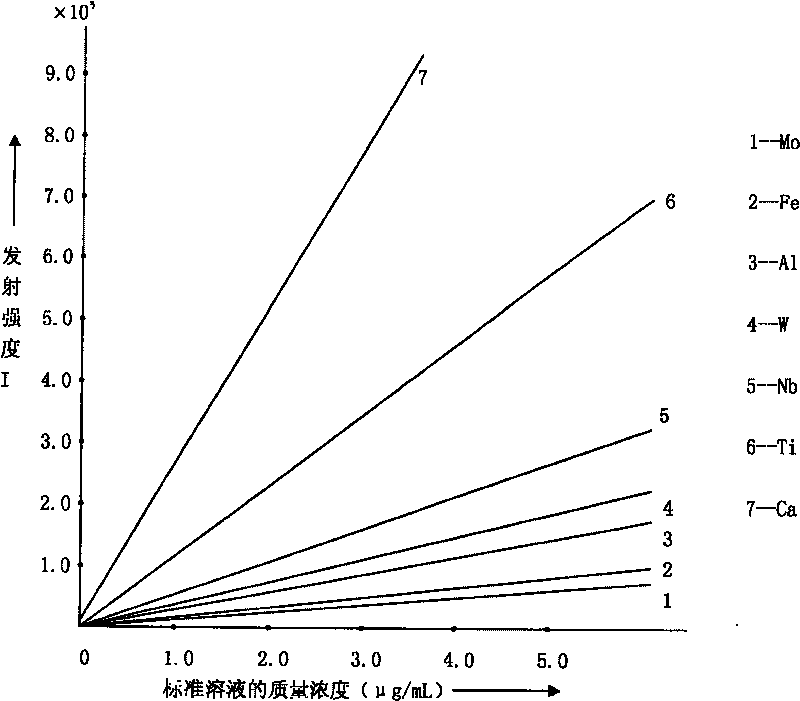
ActiveCN101303307BSolve difficult technical problemsImprove measurement accuracyPreparing sample for investigationAnalysis by thermal excitationNiobiumDecomposition
The invention discloses an analysis and detection method for impurity elements such as aluminum, calcium, ion, molybdenum, niobium, titanium, tungsten and the like in chromium carbide. The method comprises adding a chromium carbide sample into a dissolving cup, adding hydrofluoric acid, sulphuric acid and nitric acid sequentially, stirring, charging into a sealed high-pressure jar; putting the sealed high-pressure jar into a microwave extinguishing instrument for two times of microwave extinguishment; taking the high-pressure jar out of the microwave extinguishing instrument for cooling, transferring the dissolved chromium carbide liquid sample into a volumeric flask, diluting to a predetermined index, stirring; preparing a chromium substrate matched mixed standard solution series of aluminum, calcium, iron, molybdenum, niobium, titanium and tungsten; measuring element emission power of aluminum, calcium, iron, molybdenum, niobium, titanium, tungsten or the like in a blank liquid sample, a chromium carbide liquid sample and the prepared series mixed standard solution by an inductively coupled plasma atomic emission spectrometer in the same time, obtaining the analysis result by checking a standard working curve or by linear equation calculation. The invention adopts two times of microwave extinguishment using the mixed acid, solves the problem of hardness in chromium carbide decomposition, having a measurement range from 0.010% to 1.00%, which is high in accuracy, and good in precision.
Owner:ZHUZHOU HARD ALLOY GRP CO LTD
Inhibitor A detection kit and preparation method thereof
InactiveCN109061198AEliminate distractionsReduce dosageBiological testingBiotin-streptavidin complexAcridine
The invention provides an inhibitor A detection kit and a preparation method thereof to solve the problems that an existing inhibitor A detection kit is low in luminescent reaction rate, low in sensitivity, narrow in the linear range, and is high in the cost, and belongs to the technical field of immunodiagnosis. The kit comprises streptavidin magnetic bead suspension, an acridine-labeled inhibitor A antibody and biotin labeled inhibitor A antibody. The invention further provides a preparation method of the inhibitor A detection kit. The detection principle of a reagent is double antibody sandwich method. Detection is conducted by the acridine-labeled antibody and biotin labeled antibody. The luminous rate is increased and the reaction level is expanded. The specificity and detection speedof the reagent are greatly improved.
Owner:DIRUI MEDICAL TECH CO LTD
Cleaning solution for magnetic particle chemiluminescence immunoassay and preparation method thereof
InactiveCN112159733ALow background valueHigh sensitivityInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsImmune complex depositionActive agent
The invention relates to the field of biotechnology analysis, and discloses a cleaning solution for magnetic particle chemiluminescence immunoassay, which comprises an immune complex retention stabilizer, a nonionic surfactant, a buffer agent and ultrapure water, wherein the immune complex retention stabilizer is a sodium chloride solvent, the nonionic surfactant is Tween 20, and the buffer agentis a Tris-HCl buffer agent, so that the background value of a detection result can be remarkably reduced, and the detection sensitivity is improved; in addition, the invention provides a preparation method of the cleaning solution for magnetic particle chemiluminescence immunoassay, wherein the formula is simple, the raw materials are easy to obtain, and the shelf life of the prepared cleaning solution is long.
Owner:芯朗道(天津)医疗科技有限责任公司
Anti-interference electrochemical uric acid test paper and preparation method thereof
PendingCN114235925AImprove accuracyIngenious designMaterial analysis by electric/magnetic meansMetallic electrodeBlood flow
According to the anti-interference electrochemical uric acid test paper and the preparation method thereof disclosed by the invention, compared with the prior art, the anti-interference electrochemical uric acid test paper is characterized in that an anti-interference area is ingeniously designed at an inlet of a siphon pool of a reaction window, and blood firstly flows through the anti-interference area containing ascorbic acid oxidase in the siphon pool through siphoning; ascorbic acid in a blood sample passing through the anti-interference area rapidly reacts with ascorbic acid oxidase, interference of ascorbic acid is eliminated, then blood flows into the reaction area to react with urate oxidase and a water-soluble electronic mediator, and the accuracy of determining different uric acid of different individuals is improved. Meanwhile, the metal electrode is combined, electron transfer is accelerated, current is reduced into a detection signal, detection can be carried out at a low negative potential, background current is further reduced, sensitivity is improved, and the detection time can be shortened to be within 10 seconds. By combining with the interference-removing enzyme layer, the ascorbic acid removing effect is remarkable, the sensitivity is high, and the accuracy is good.
Owner:NANJING EAGLENOS CO LTD
Reagent card for detecting therapeutic effect of thienopyridine antiplatelet drugs and application method and application of reagent card
ActiveCN105717263AHas practical valueEliminate distractionsBiological testingTesting medicinal preparationsMicrosphereFreeze-drying
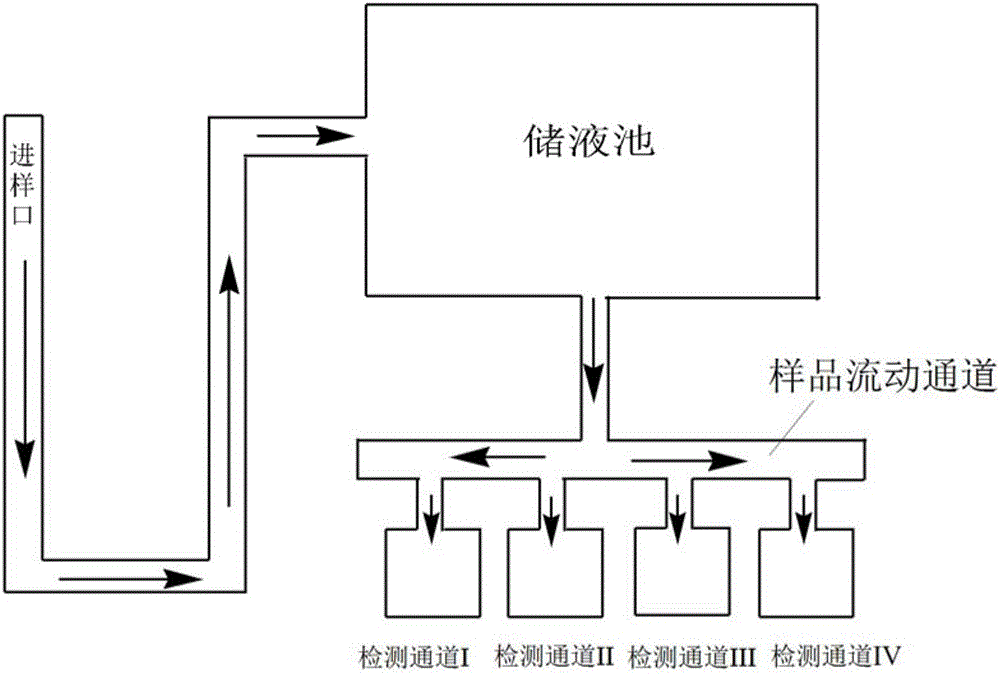
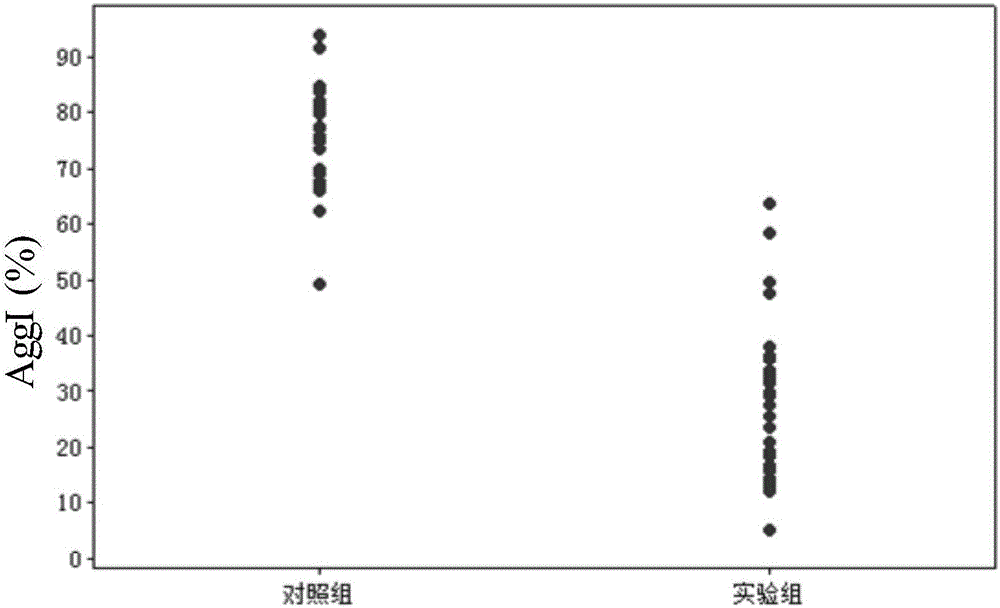
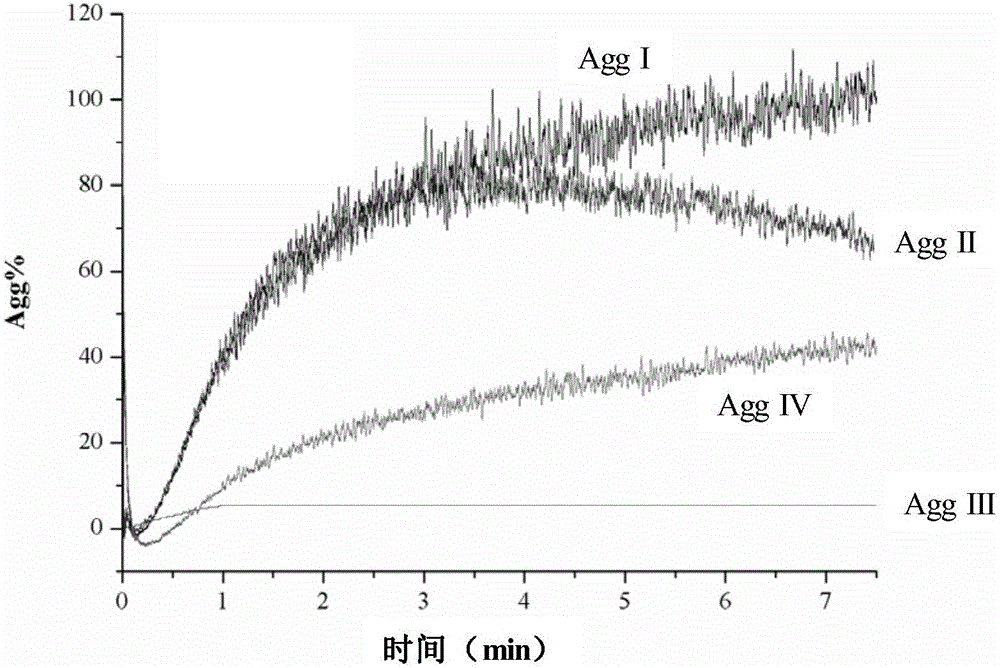
The invention provides a reagent card for detecting the therapeutic effect of thienopyridine antiplatelet drugs and an application method and application of the reagent card. The reagent card comprises four detection channels which are sequentially arranged in parallel and are independent of one another, the detection channel I contains channel I detection reagent, and the channel I detection reagent contains a lyophilized agent, wherein the lyophilized agent is obtained by firmly bonding activated dye microspheres with receptor ligand of blood platelets GpIIb / IIIa through amido bonds, then adding blood coagulation factors, and conducting freeze-drying; the detection channel II contains channel II detection reagent, and the channel II detection reagent contains a lyophilized agent serving as a blood platelet maximum activating agent; the detection channel III contains no detection reagent, the detection channel IV contains channel IV detection reagent, and the channel IV detection reagent contains a lyophilized agent serving as a blood platelet P2Y12 receptor activating agent and P2Y1 receptor antagonist. By means of the reagent card, the therapeutic effect of thienopyridine antiplatelet drugs can be detected easily, fast, conveniently and accurately.
Owner:北京乐普诊断科技股份有限公司
Antibody horseradish peroxidase marker and preparation and application thereof
PendingCN112924665ASpontaneous NHS responseLong-term effective preservationBiological testingAntigenAbzyme
The invention discloses an antibody horseradish peroxidase marker, which is formed by cross-linking an amino-modified antibody (NH2-Ab) and a sulfydryl-modified horseradish peroxidase (SH-HRP), and the antibody can be any antibody suitable for an antigen enzyme-linked immunosorbent assay kit or a chemiluminescence detection kit. The invention also discloses an application of the marker in preparation of an antigen enzyme-linked immunosorbent assay kit or a chemiluminescence detection kit. Experiments prove that when the antibody horseradish peroxidase marker is combined with an enzyme stabilizer and a diluent for use, the sensitivity and the stability of the antibody enzyme marker can be remarkably improved, the detection background value is greatly reduced, the specificity is better, and the application prospect is great.
Owner:山东莱博生物科技有限公司
Fusion protein antigen for diagnosing dog brucellosis
ActiveCN112574320ARemain solubleOvercome securityBacteriaMicroorganism based processesEscherichia coliBrucella
The invention provides a brucellosis specific fusion protein antigen. The amino acid sequence of the brucellosis specific fusion protein antigen is shown as SEQ ID No: 1. The fusion protein antigen provided by the invention comprises a plurality of brucella dominant outer membrane epitopes, and has good extensibility, stability and biological activity. The canine brucellosis ELISA antibody detection kit established by using the fusion protein provided by the invention has high sensitivity and specificity. Specificity cross experiments show that the OD450 ratio of the fusion protein to escherichia coli O157 serum, escherichia coli H7 serum, salmonella O antigen multivalent serum, yersinia small intestine serum and listeria monocytogenes serum to negative serum is smaller than 1.5, and the OD450 ratio of brucella canis positive serum to negative serum is larger than 5; therefore, it shows that the fusion protein provided by the invention does not have cross reaction with serum infected with other bacteria, so that the detection accuracy is ensured.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Acid-alkali generation device without requirement for degassing
PendingCN109763136AHigh purityLow background valueCellsElectrolytic organic productionIon chromatographyElectrolysis
The invention discloses a high and low pressure acid-alkali generation device without a requirement for degassing or a requirement for additionally providing a solution driving unit. The device is widely applied in chemical analysis, and particularly provides a very convenient working mode for ion chromatography and potentiometric titration. A principle of the acid-alkali generation device is thattwo cavity bodies are utilized, electrodes and a high-pressure acid-alkali generation cavity are separated by an ion exchange membrane group, H2 and O2 which are generated by electrolysis of two cavities cannot enter the acid or alkali generation cavity body in the high-pressure cavity, so that a high-pressure degassing part is omitted; and through such structure, not only is cost of an instrument reduced, but also a fault rate of the instrument is reduced. The invention discloses three devices formed by a method, i.e., 1, a three-cavity-body double-liquid-storage-tank acid-alkali generationdevice; 2, a three-cavity-body single-liquid-storage-tank acid-alkali generation device; and 3, a five-cavity-body single-liquid-storage-tank acid-alkali simultaneous generation device.
Owner:BEIJING LIYUAN INSTR CO LTD
Method for detecting silicon dioxide in foods and food additives
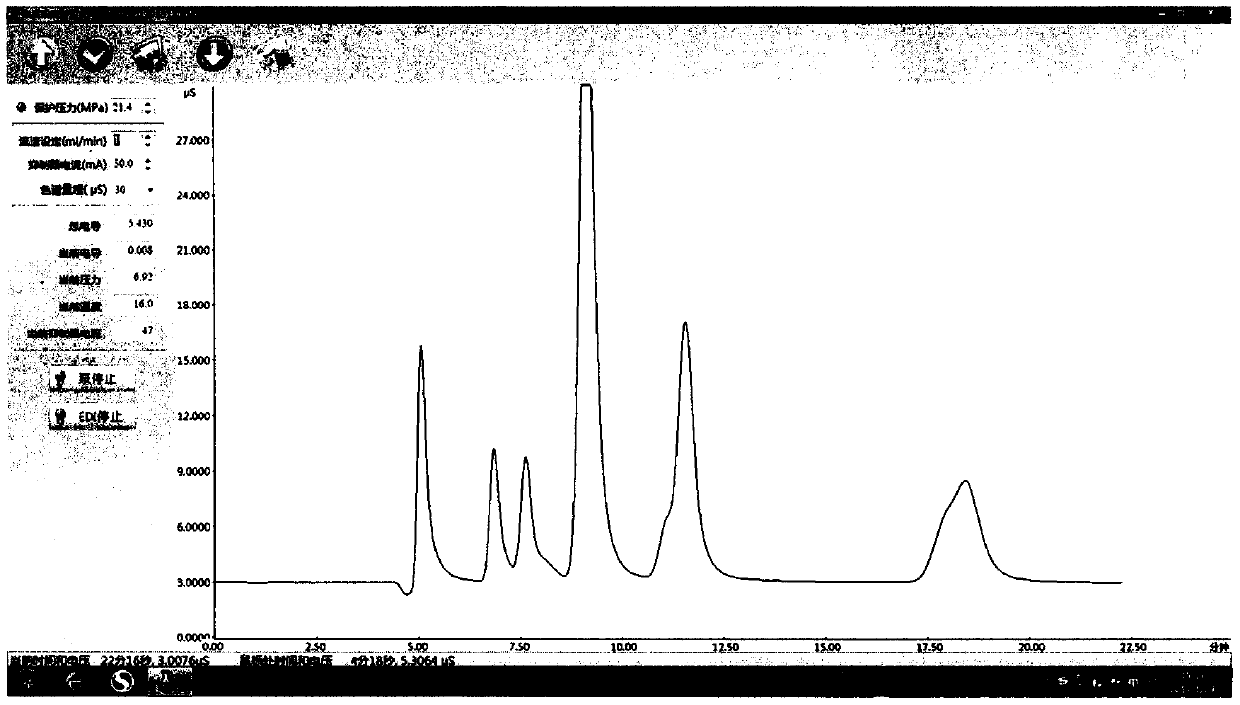
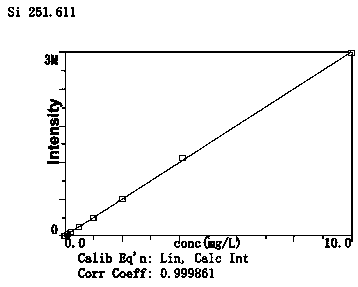
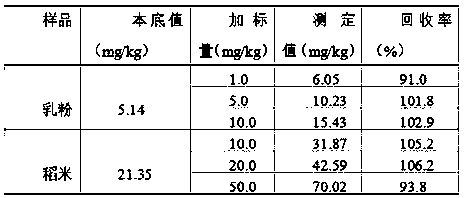
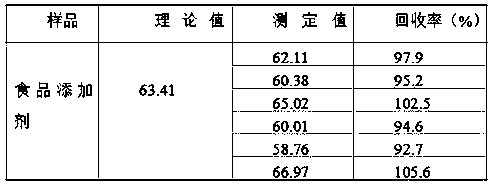
InactiveCN108802012AEliminate distractionsShorten the timePreparing sample for investigationAnalysis by thermal excitationFood additivePotassium hydroxide
The invention belongs to the technical field of food analysis, and particularly relates to a method for detecting silicon dioxide in foods and food additives. The method comprises the following steps:weighing a sample, carrying out microwave digestion on the sample with nitric acid and hydrogen peroxide, filtering after digestion, and washing with water; adding nitric acid, hydrochloric acid, hydrofluoric acid for microwave digestion, and adding potassium hydroxide to a solution for reaction after digestion, thereby obtaining a to-be-tested sample solution; measuring with the sample solutionby an inductively coupled plasma emission spectrometer; measuring the intensity of silicon in a solution of a standard curve and the solution to be tested by the plasma emission spectrometer, calibrating the curve, and calculating the content of silicon dioxide in the sample to be tested. The method provided by the invention has the advantages of high digestion speed, good digestion effect and high recovery rate, and can deduct the influence of background, so that detection data is accurate and reliable; potassium hydroxide added to the solution after digestion can neutralize part of acid, thereby reducing the damage of acid to instruments. The method is suitable for determination of silica in the foods and the food additives.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com