Method for detecting combination of polypeptide medicine and plasma proteins
A plasma protein and drug technology, applied in the field of life science, can solve the problems of small dosage, strong interference of endogenous substances, difficult handling, etc., and achieve the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
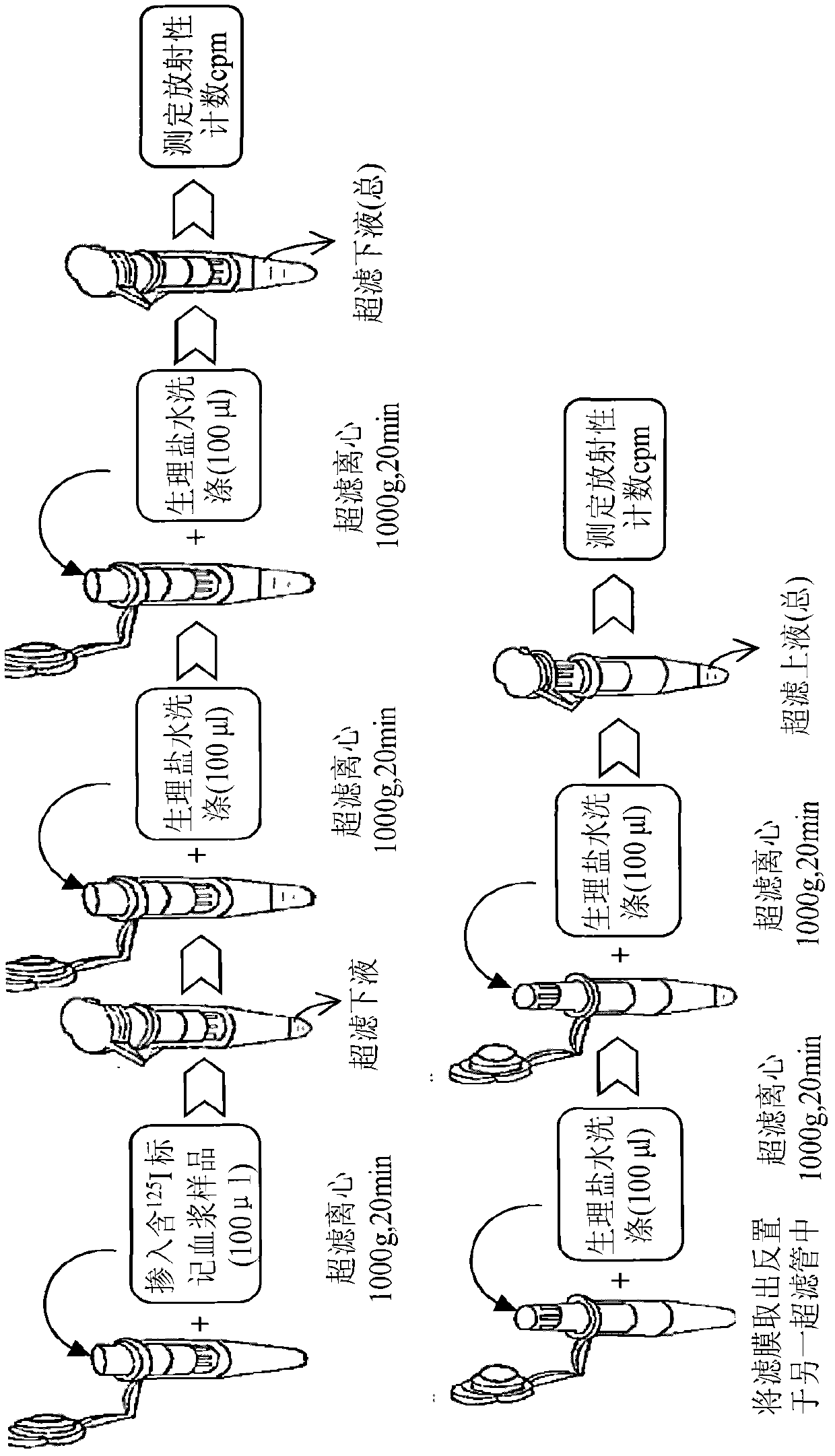
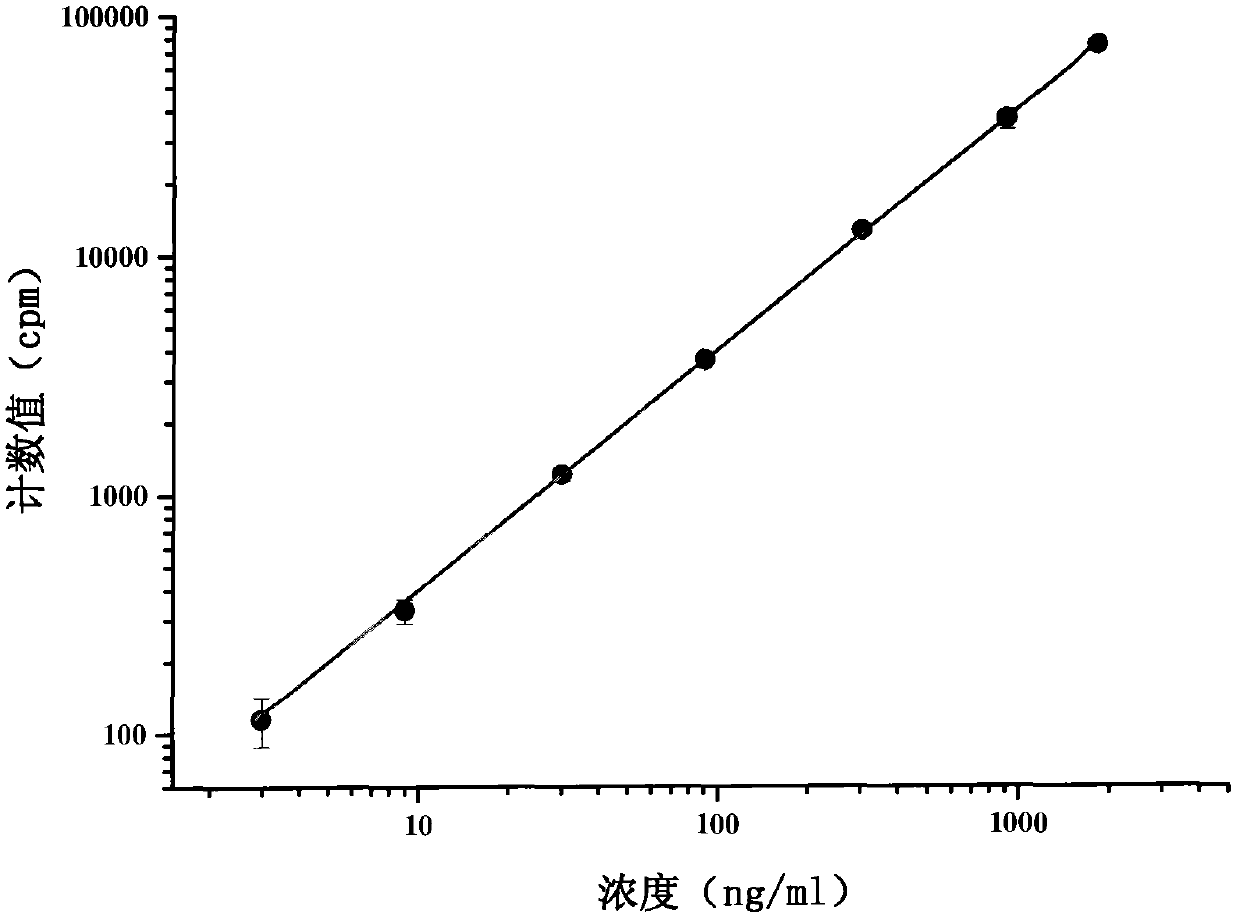
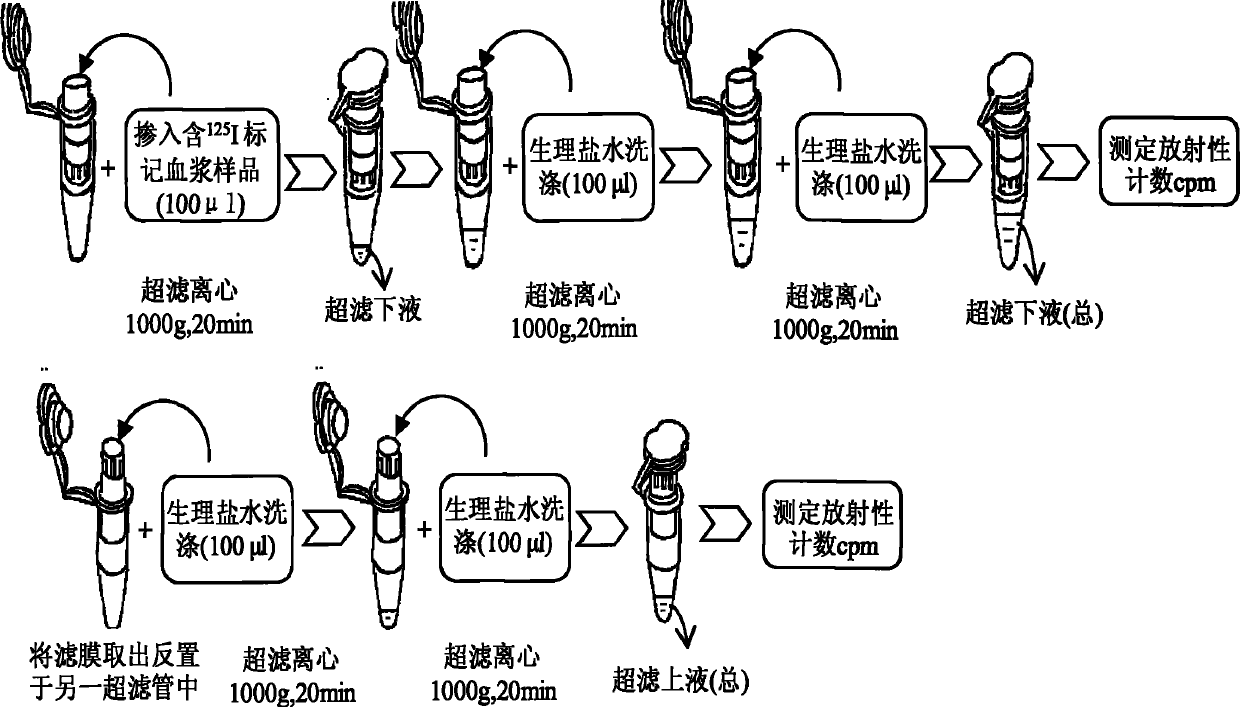
[0037] In this example, the method of combining isotope tracing, centrifugation and ultrafiltration technology established in the present invention was used to determine the drug protein binding rate of recombinant human thymosin α1 (rh-Tα1) in rat plasma. The specific analysis method is as follows:
[0038] 1. Use chloramine T method (Ch-T) to label recombinant human thymosin α1 (rh-Tα1) to prepare 125 I-rh-Tα1 freeze-dried product. rh-Tα1 is produced by Shenzhen Neptunus Pharmaceutical Co., Ltd., with batch number 20050301. The sample is a white freeze-dried powder with a purity of 98.51% and a content of 1.5 mg / bottle. The sample was stored at 4°C before the test. 5μg rh-Tα1 was added to 20μl 0.1M pH 7.4 phosphate buffer, the final concentration was 0.25μg / μl. Then add 2.25mCi Na 125 I and 15μg of Ch-T (0.5μg / μl), after reacting for 1min, immediately add 30μg of Na 2 S 2 O 5 (1μg / μl) to stop the reaction. Separate and purify the reaction mixture on a C18 column to obtain 125...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com