Traditional Chinese medicine pharmacokinetics-pharmacodynamics combined analysis method
A joint analysis and pharmacokinetics technology, applied in the direction of analytical materials, scientific instruments, material separation, etc., can solve the problems that the effects cannot be directly and continuously quantitatively measured, the research and application of PK-PD are limited, and the mechanism of action is not very clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Take 15 SD rats and randomly divide them into 3 groups: blank group, model group and model administration group. In the model group and the model administration group, the myocardial ischemic disease model was created by ligation of the left anterior descending coronary artery. The model administration group was given Xuesaitong injection, 18mg / kg, subcutaneously in the lower abdomen of the model 7 days after the model was established. Plasma was collected from blank group, model group and model administration group for future use. The spectrum of endogenous metabolites in plasma was determined by GC-MS (see attached figure 1 , where a is the blank group, b is the myocardial ischemic disease model group), first compare the changes in the metabolite profiles of the blank group and the model group, find out the metabolite groups with significant abnormal changes in the model group, and find 24 disease biomarkers, Lactic acid, 3-phosphoglycerol, glyceric acid, succini...
Embodiment 2
[0041] According to the method of Example 1, the difference lies in the PK-PD joint analysis of the component of Xuesaitong injection notoginseng saponin R1
[0042] The plasma concentration-time curve of notoginseng saponin R1 and the time-effect curves of the above seven pharmacodynamic markers were analyzed by Pearson correlation. The correlation coefficient (r) results are shown in Table 3. The negative correlation coefficient shows that the blood concentration of notoginseng saponin R1 has a negative correlation with the regulatory effect on the efficacy markers, that is, the larger the negative correlation coefficient is, the more the callback effect of notoginseng saponin R1 on the corresponding markers plays a major role. Therefore, it can be seen from the table that notoginseng saponin R1 has a callback effect on lactic acid, succinic acid, malic acid, and cholesterol, especially has a strong regulatory effect on lactic acid. It can be seen that R1 is one of the main ...
Embodiment 3
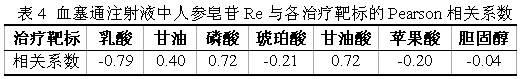
[0045] According to the method of Example 1, the difference lies in the PK-PD joint analysis of ginsenoside Re, a component of Xuesaitong injection
[0046] Pearson correlation analysis was performed on the plasma concentration-time curve of ginsenoside Re and the time-effect curve of the above-mentioned seven pharmacodynamic markers. The correlation coefficient (r) results are shown in Table 4. The negative correlation coefficient shows that the blood concentration of ginsenoside Re has a negative correlation with the regulatory effect on the efficacy markers, that is, the larger the negative correlation coefficient is, the more dominant the callback effect of ginsenoside Re is on the corresponding markers. Therefore, it can be seen from the table that ginsenoside Re has a callback effect on lactic acid, succinic acid, malic acid, and cholesterol, especially has a strong regulatory effect on lactic acid. It can be seen that Re is one of the main medicinal ingredients, and its...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com