Tumor targeting polypeptide and application thereof in preparation of polypeptide drug conjugate
A drug conjugate, tumor targeting technology, applied in the field of anti-tumor, can solve the problem of difficulty in coupling antibodies and effector molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
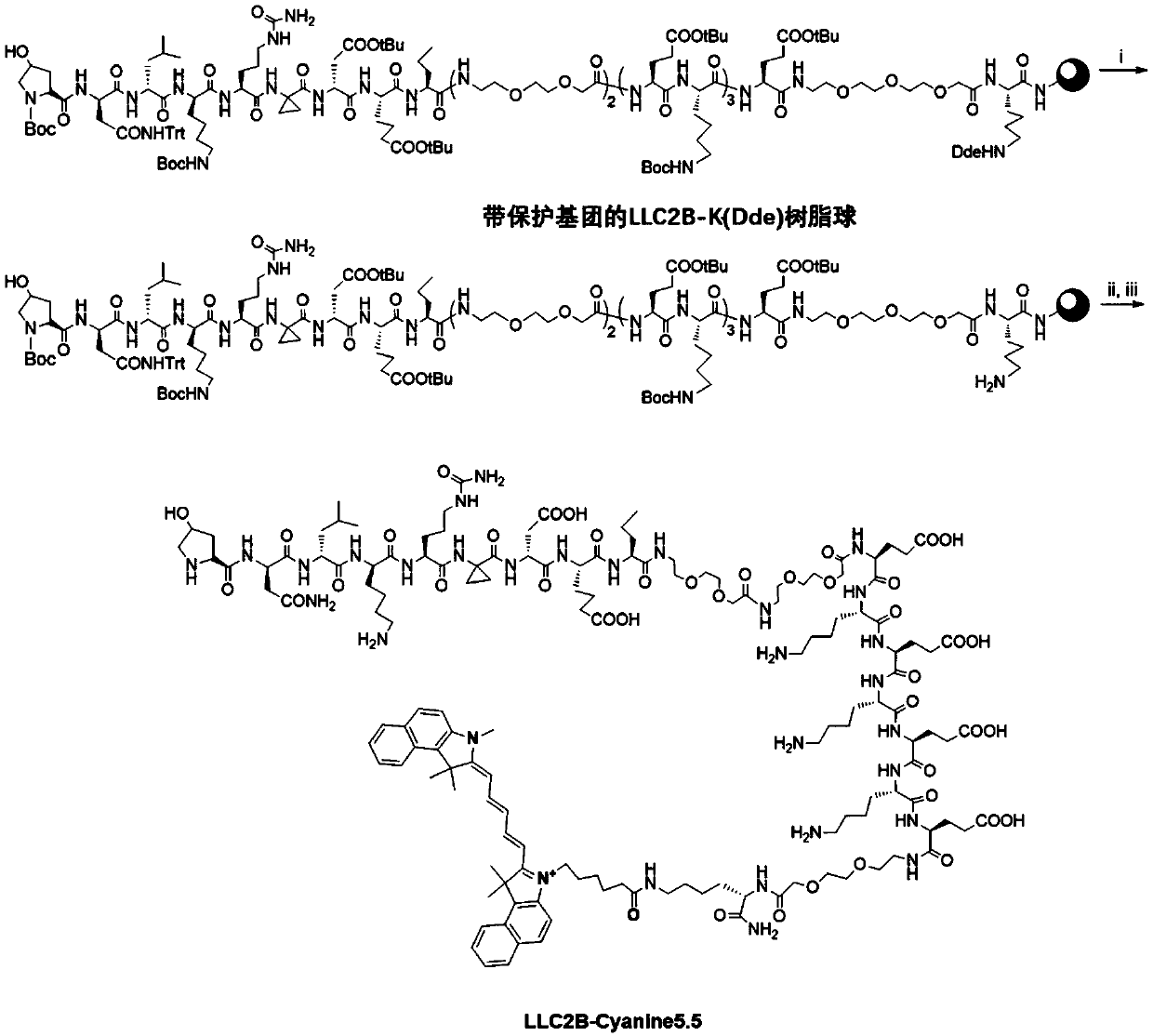
[0076] The synthesis of embodiment 1, LLC2B
[0077] The sequence (Hyp)-(D-Asn)-(D-Leu)-D-Lys)-(Cit)-(Acpc)-(D-Asp)-(Aad)-(Nva) is the synthesis and conventional polypeptide of LLC2B The synthesis method is similar, as follows:
[0078](1) Weigh the unnatural amino acid containing the Fmoc protection group according to the following formula: grams of amide resin × degree of substitution of amide resin × 4 times molar concentration × molecular weight of a certain Fmoc-amino acid. 6-Cl HOBt and DIC are also weighed according to the following formula: grams of amide resin × degree of substitution of amide resin × 4 times molar concentration × molecular weight. After weighing 6-Cl HOBt and DIC, add analytical grade DMF solution, that is, the mass of HOBt in 1ml solution = 0.05 × 0.31 × 4 × 169.7 = 10.52 mg; the volume of DIC = 0.05 × 0.31 × 4 × 126.2 / 0.815 = 96 μl, and then add the weighed non-natural amino acid containing Fmoc protection group (in operation, it can be prepared ...
Embodiment 2
[0084] Example 2: Affinity detection results of LLC2B and RTK family members
[0085] (1) Prepare sterilized and filtered ultrapure water as the sample solvent, dissolve the small molecule polypeptide (LLC2B) and protein (FGFR2 protein and HER2 protein) and then store in a -80°C refrigerator; among them, the FGFR2 protein and HER2 protein were purchased from Beijing Sino Biological Technology Co., Ltd.;
[0086] (2) Configure the pre-set small molecule concentration and protein concentration, and the ratio of small molecule polypeptide to protein concentration is 80 to 150 times (this concentration ratio is the concentration ratio of μM level);
[0087] (3) After the sample is prepared, it is filtered, and it is placed at room temperature for 30 minutes to be operated on the machine (the instrument used is an isothermal titration calorimeter ITC200)
[0088] (4) Manually clean the sample pool and reference pool for 7 to 8 times, and then machine clean the sample pool and titr...
Embodiment 3
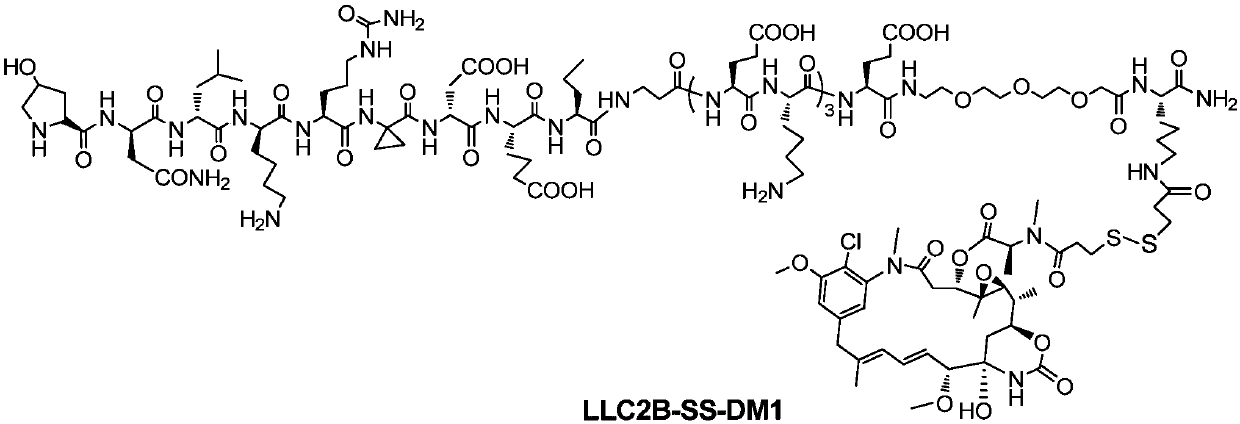
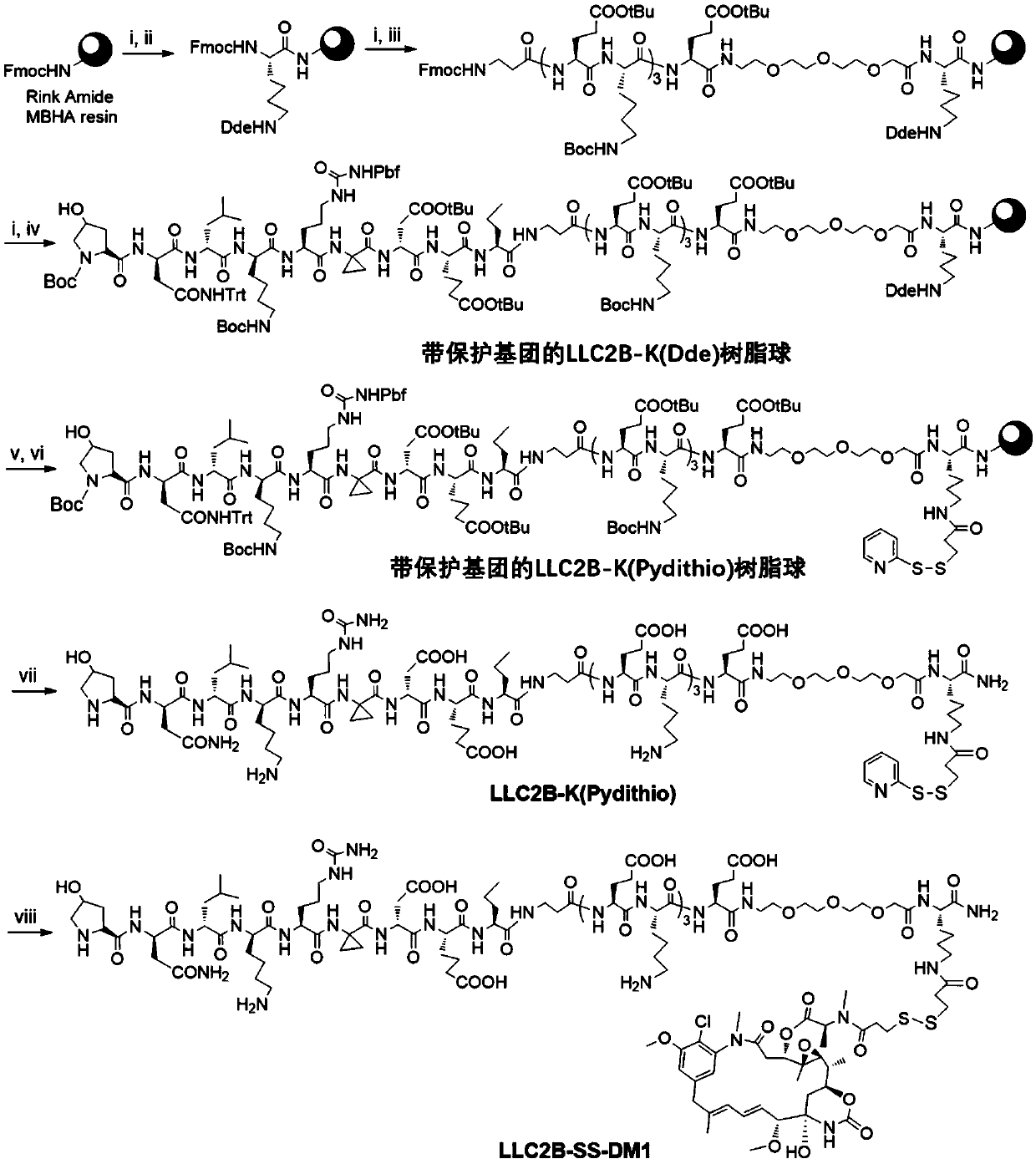
[0094] Example 3: Synthesis of LLC2B-SS-DM1
[0095] The structure of LLC2B-SS-DM1 is as figure 1 As shown, the coupling process is as figure 2 Shown:
[0096] (1) Weigh 1.0 g of amide resin, then put the amide resin into the reaction tube, add 2 times the volume of DMF (N,N-dimethylformamide) and soak for 2 to 3 hours; discard the solution by suction, add 20% (w / w) DMF solution (8 milliliters) of 4-methylpiperidine, reacted for 5 minutes, removed the solution and added DMF solution (8 milliliters) containing 20% (w / w) 4-methylpiperidine ), and reacted for 15 minutes; the solvent was drained through a suction filtration device, followed by washing with DMF three times, methanol three times and DMF three times, and then adding analytical grade DMF to balance for 2 to 5 minutes.
[0097] (2) Drain the solvent through a suction filtration device, add 2 times the volume of the compound Fmoc-Lys(Dde)-OH and 5 times the molar concentration of HCTU containing 5 times the molar ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com