Pharmaceutical composition containing pregabalin with improved stability and method for preparing same
A composition and stability technology, which is applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as unstable structural characteristics, inability to ensure stability, and stability problems, so as to improve compliance , Improve dosage convenience and ensure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
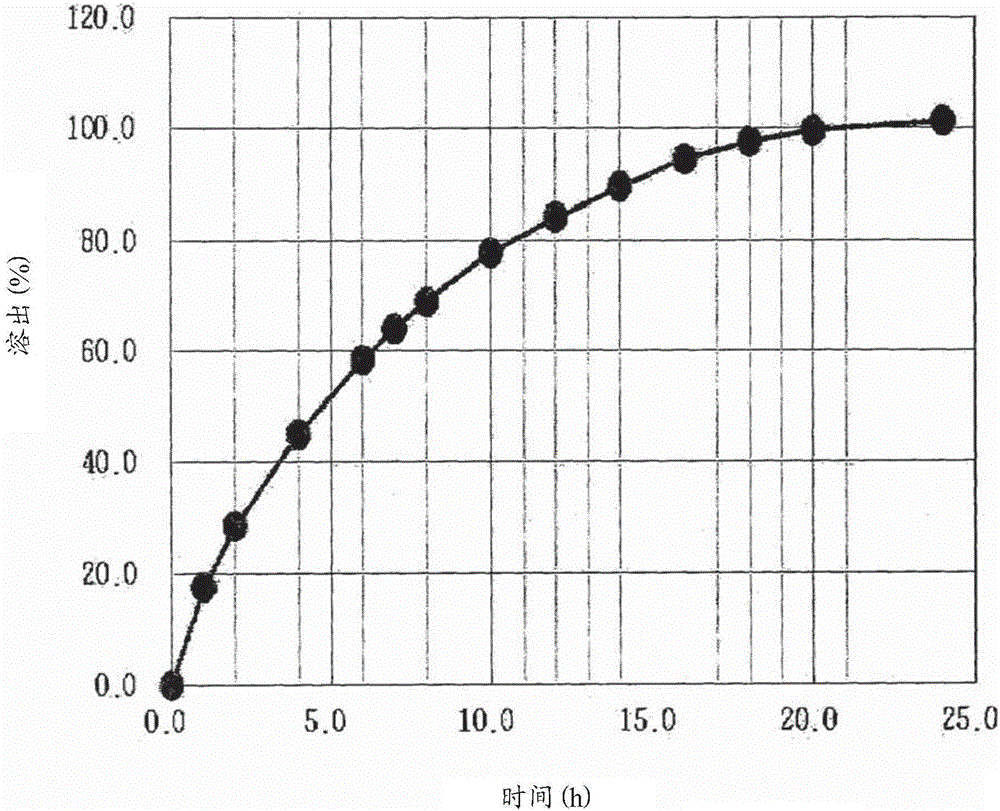
[0023] The present inventors repeated and verified various prior art related to sustained release formulations of pregabalin. As a result, they found that because of the poor compatibility between pregabalin and various excipients, adding various excipients directly reduced the stability and increased the generation of related compounds. Therefore, ensuring that the main ingredient pregabalin The stability of Bahrain is the prerequisite for providing sustained-release preparations of pregabalin. When the stability of pregabalin is ensured to enhance its compatibility with excipients, a once-a-day formulation that remains in the upper part of the gastrointestinal tract for a longer period of time can finally be obtained, thereby completing the present invention.
[0024] The sustained-release pharmaceutical composition according to the present invention is formulated by forming a coating unit on the outer surface of a drug unit containing an active ingredient, and then mixing t...
preparation example 1-1
[0063] Preparation Example 1-1: Preparation of active ingredient-containing coating unit (1)
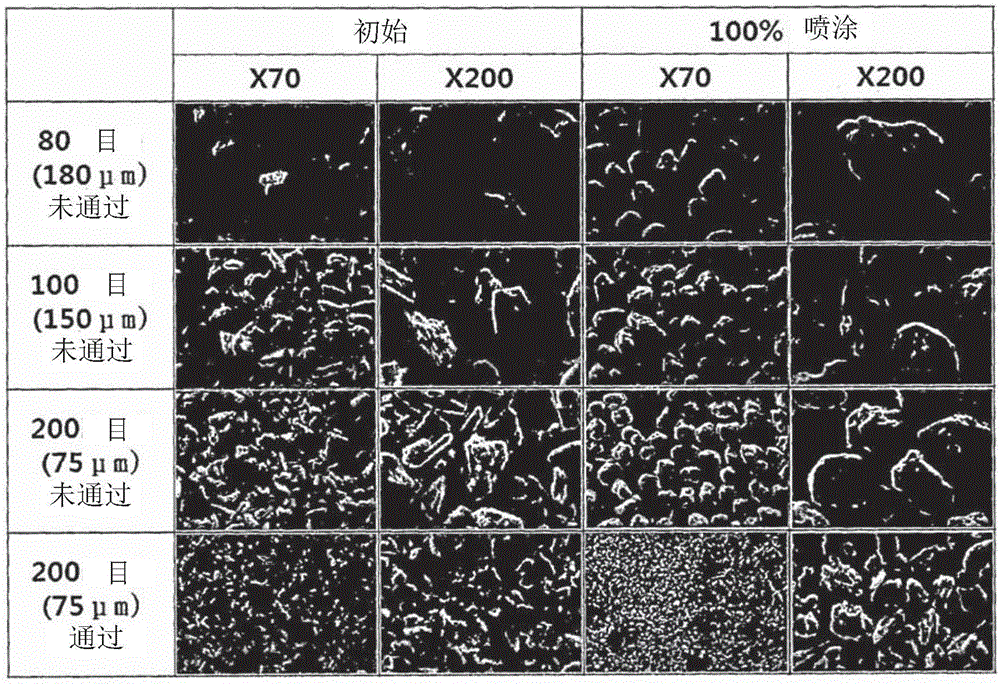
[0064] To prepare coated units containing the active ingredient (pregabalin), first 56 mg of mannitol and 24 mg of Kollicoat IR were completely dissolved in water to prepare a coating. The coating agent powder was used to coat 300 mg of pregabalin to prepare a pregabalin-coated unit with strong stability. In this regard, powder coating of pregabalin was performed using a fluidized bed coater (GlattGPCG2Labsystem, Germany) and operated under the following conditions: input air temperature 65 °C, product bed The temperature was 30 °C, the feed rate was 1.34 mL / min, the nozzle pressure was 1.5 bar and the nozzle diameter was 0.8 mm. In Table 1 the operating conditions of the fluidized bed coater are given.
[0065] [Table 1]
[0066]
[0067] The fluidized bed conditions in Table 1 are descriptions of the detailed conditions and preparation methods of the present invention, and ...
preparation example 1-2
[0068] Preparation Example 1-2: Preparation of Active Ingredient-Containing Coating Unit (2)
[0069] [Table 2]
[0070] raw material
[0071] Under the conditions of table 1, make 300g pregabalin fluidized in the fluidized bed coater, spray coating agent then; is 14.7wt%) and 24g of Kollicoat IR were completely dissolved in 1,176g of purified water to prepare a coating agent. This prepares a coated unit containing the main ingredient. Table 2 gives the ingredients used in their amounts and in wt% in the coated units thus formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com