Pharmaceutical composition containing 5'-Ara-C-O-amino ester
A technology of amino acid esters and cytarabine, which is applied in the direction of drug combinations, medical preparations containing active ingredients, antineoplastic drugs, etc. poor permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] tablet
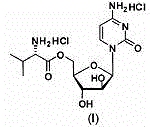
[0034] Cytarabine 5'-0-L-valine ester hydrochloride 30g lactose 60g microcrystalline cellulose 25g Crospovidone 15g citric acid 1g Magnesium stearate 2g production 1000 pieces
[0035] Preparation Process
[0036] Pass cytarabine 5'-0-L-valine ester hydrochloride through a 80 mesh sieve, mix the main ingredient with other excipients except magnesium stearate, add appropriate amount of water, make soft material, and granulate with 18 mesh , dried at 60 degrees, 16 whole grains, mixed with magnesium stearate, and compressed into tablets.
Embodiment 2
[0038] tablet
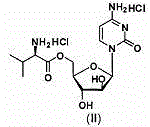
[0039] Cytarabine 5'-0-D-valine ester hydrochloride 15g Mannitol 60g pregelatinized starch 25g Cross-linked polyvinylpyrrolidone 15g tartaric acid 1.5g Magnesium stearate 2g production 1000 pieces
[0040] Preparation process: with embodiment 1
Embodiment 3
[0042] tablet
[0043] Cytarabine 30g lactose 60g hypromellose 25g Sodium carboxymethyl starch 15g citric acid 1g Magnesium stearate 2g production 1000 pieces
[0044] Preparation process: with embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com