Patents
Literature
303 results about "Hypotension shock" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Decreased cardiac output despite normal blood volume, due to severe congestive heart failure, large myocardial infarction, heart valve problems, or extremely low heart rate (bradycardia), often produces hypotension and can rapidly progress to cardiogenic shock. Arrhythmias often result in hypotension by this mechanism.
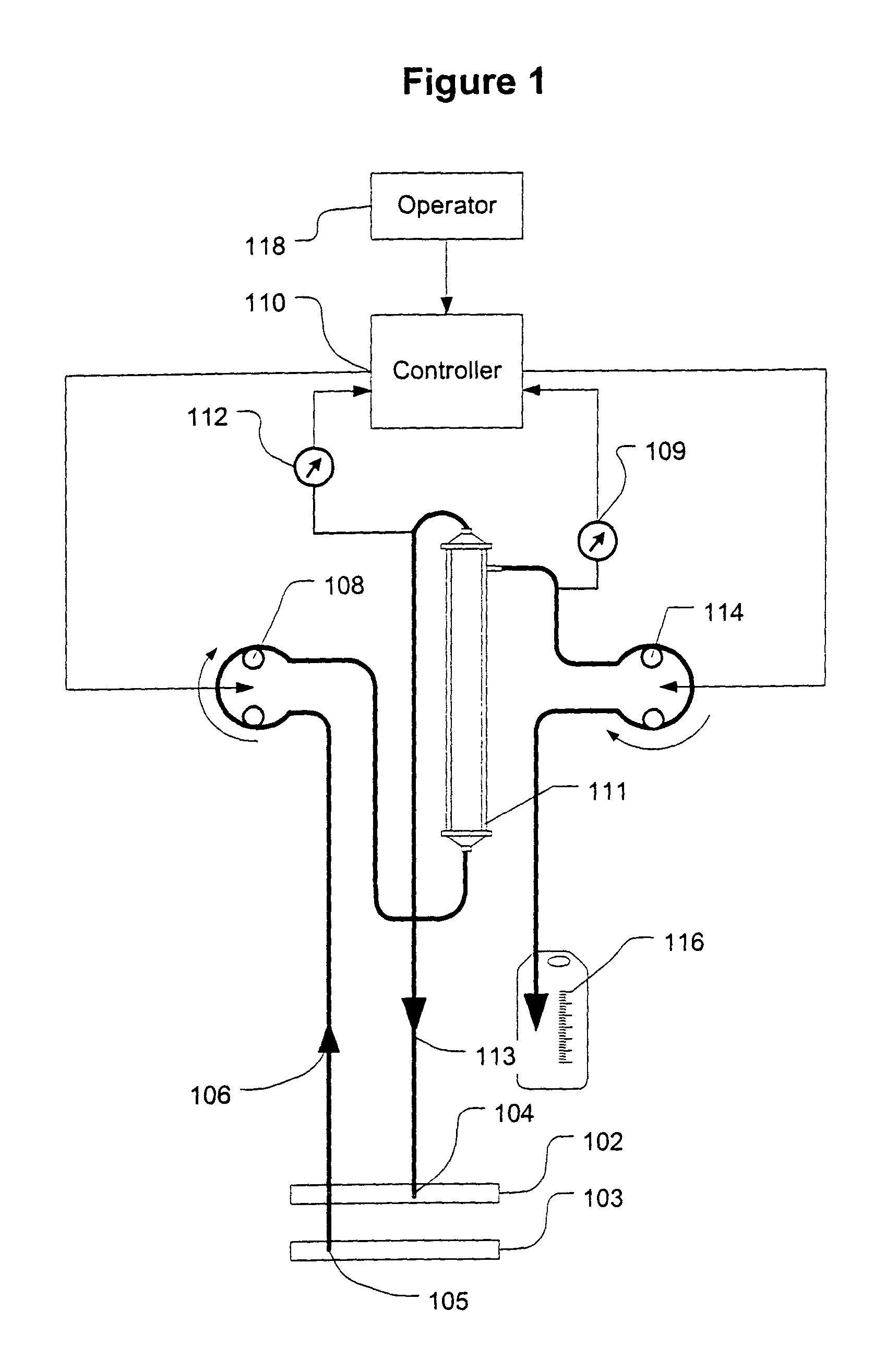
Systems and methods for hypotension
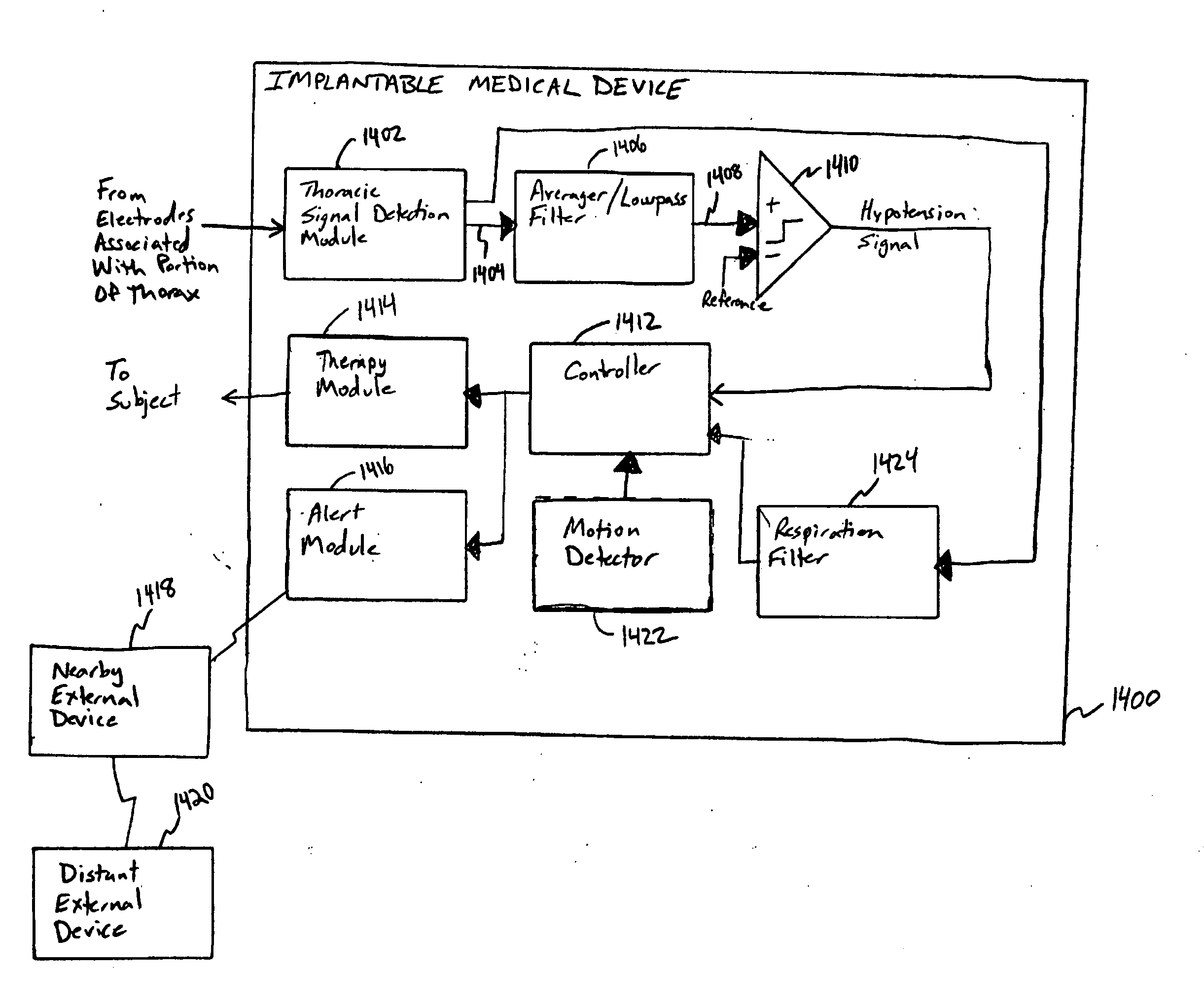
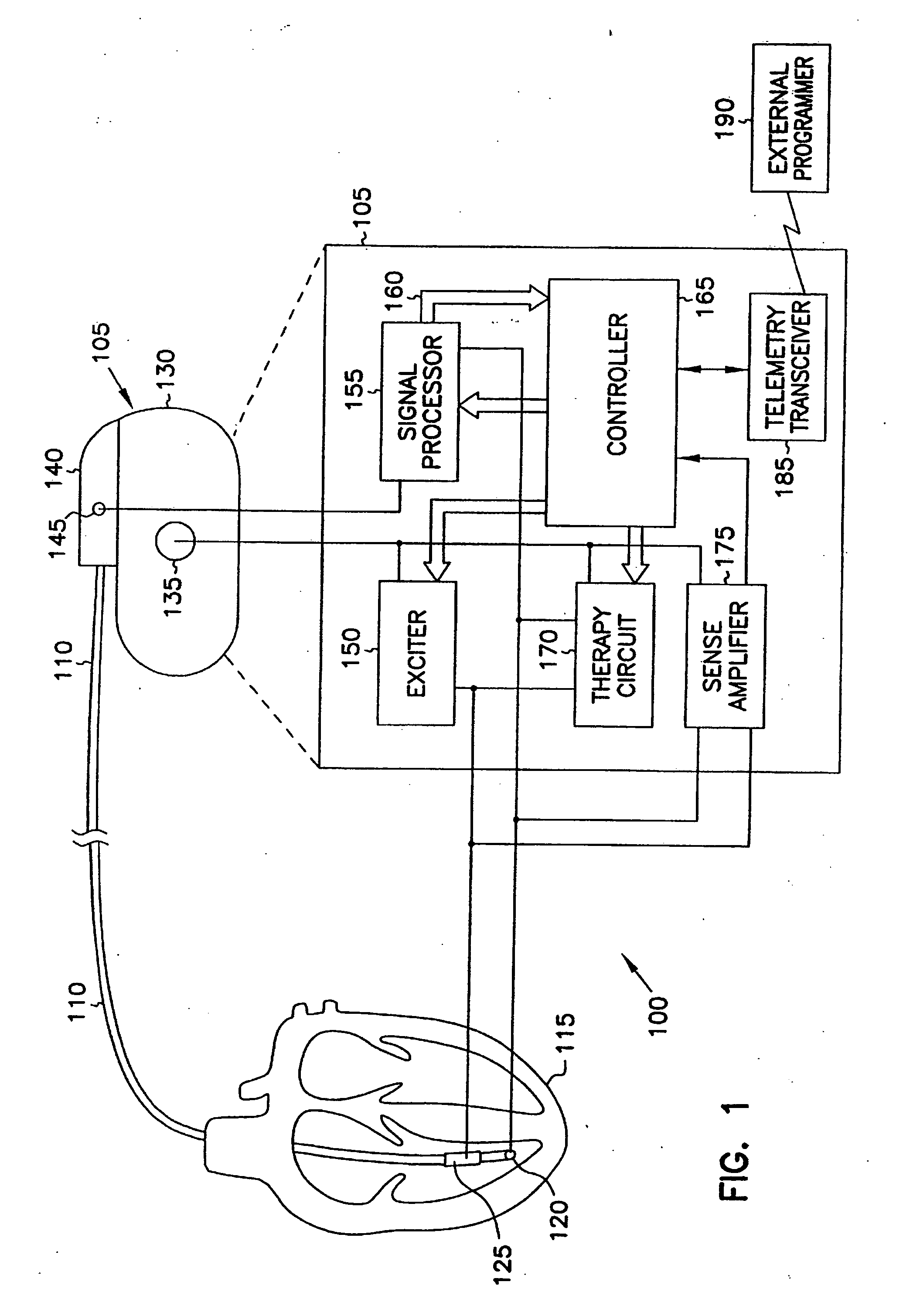
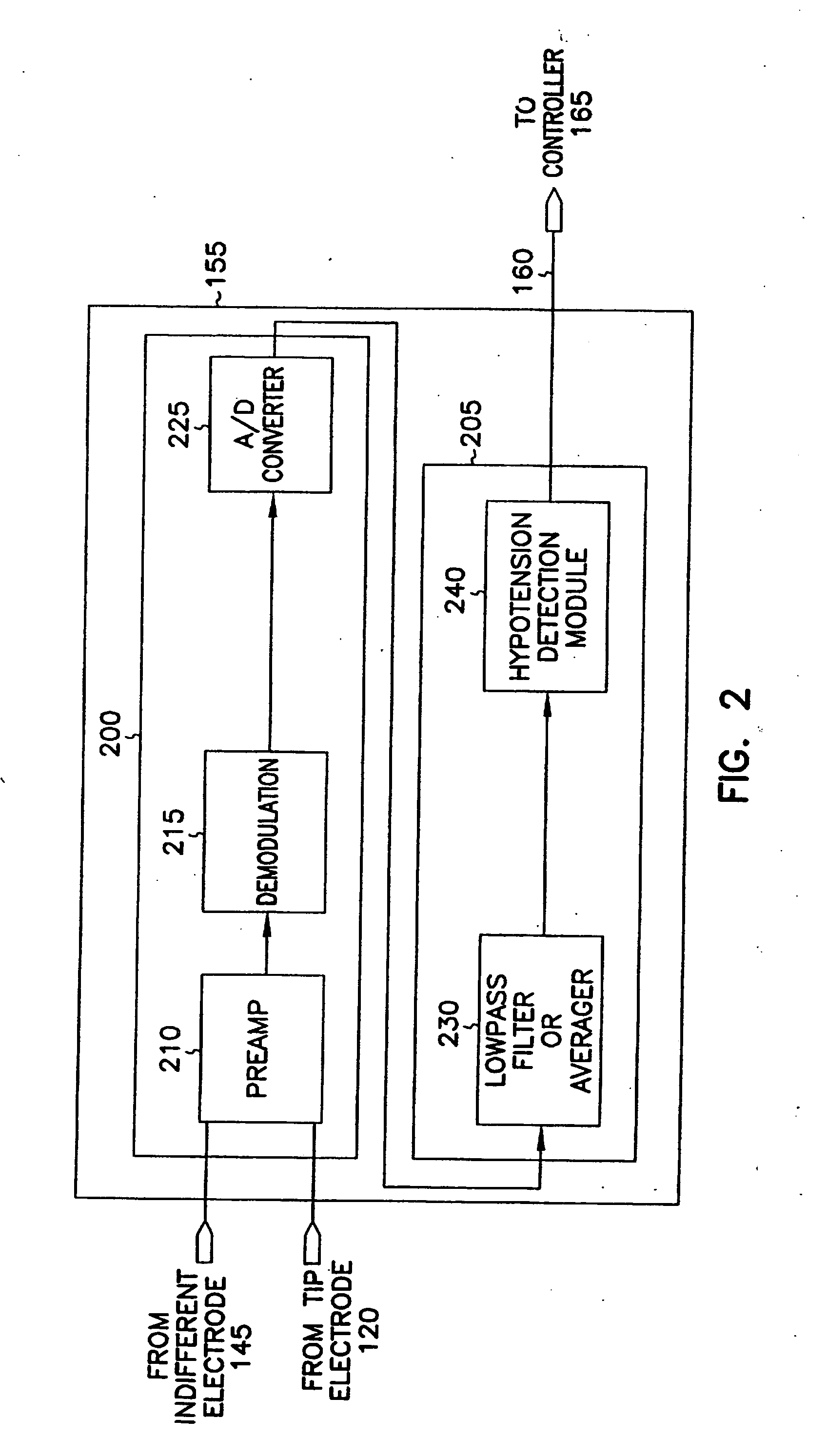
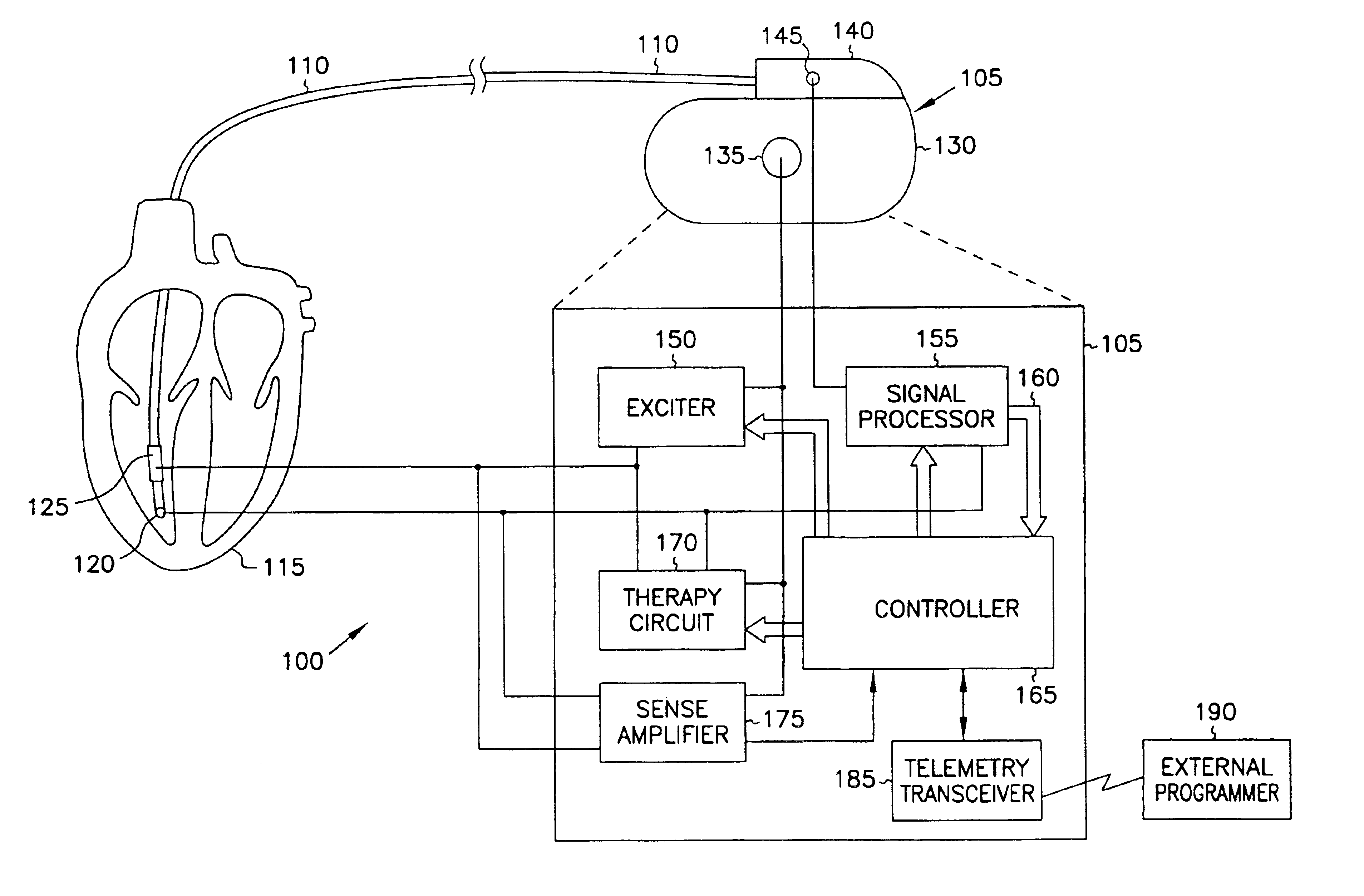
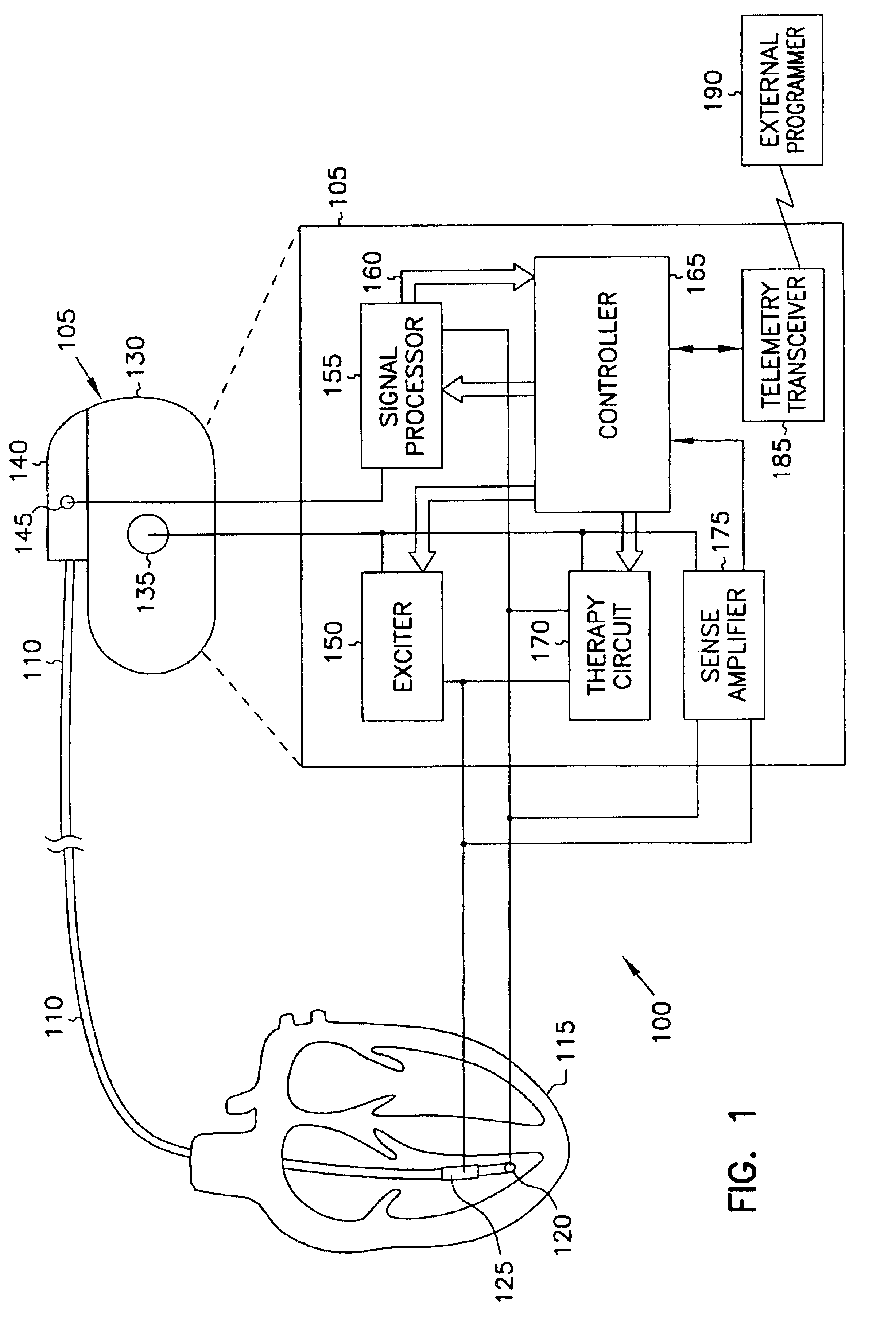
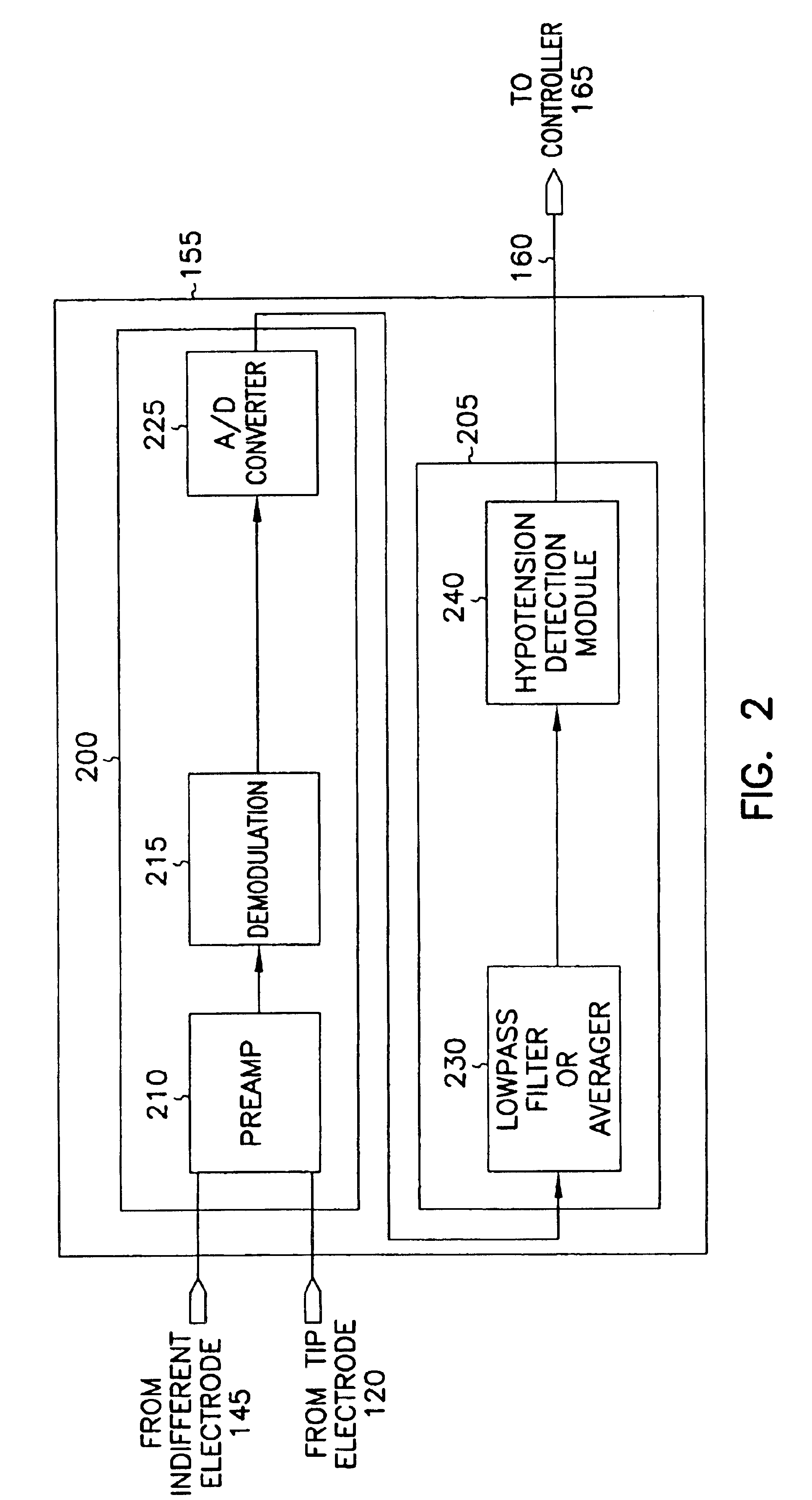
This document discusses, among other things, systems and methods that detect hypotension based on a measurement of thoracic impedance. It also provides an alert, a logging, or a therapy to treat the hypotension. Examples of anti-hypotension therapies include, among other things, pacing therapy, neural stimulation therapy, drug infusion therapy, or gene therapy.
Owner:CARDIAC PACEMAKERS INC
Cardiac rhythm management system for hypotension
A cardiac rhythm management system detects hypotension based on a measurement of thoracic impedance. It also provides therapy to treat the hypotension.
Owner:CARDIAC PACEMAKERS INC
Methods and compositions for the treatment and diagnosis of diseases characterized by vascular leak, hypotension, or a procoagulant state
InactiveUS20070154482A1Improve subject 's conditionIncrease cardiac outputCompounds screening/testingOrganic active ingredientsHigh dose therapyHigh doses
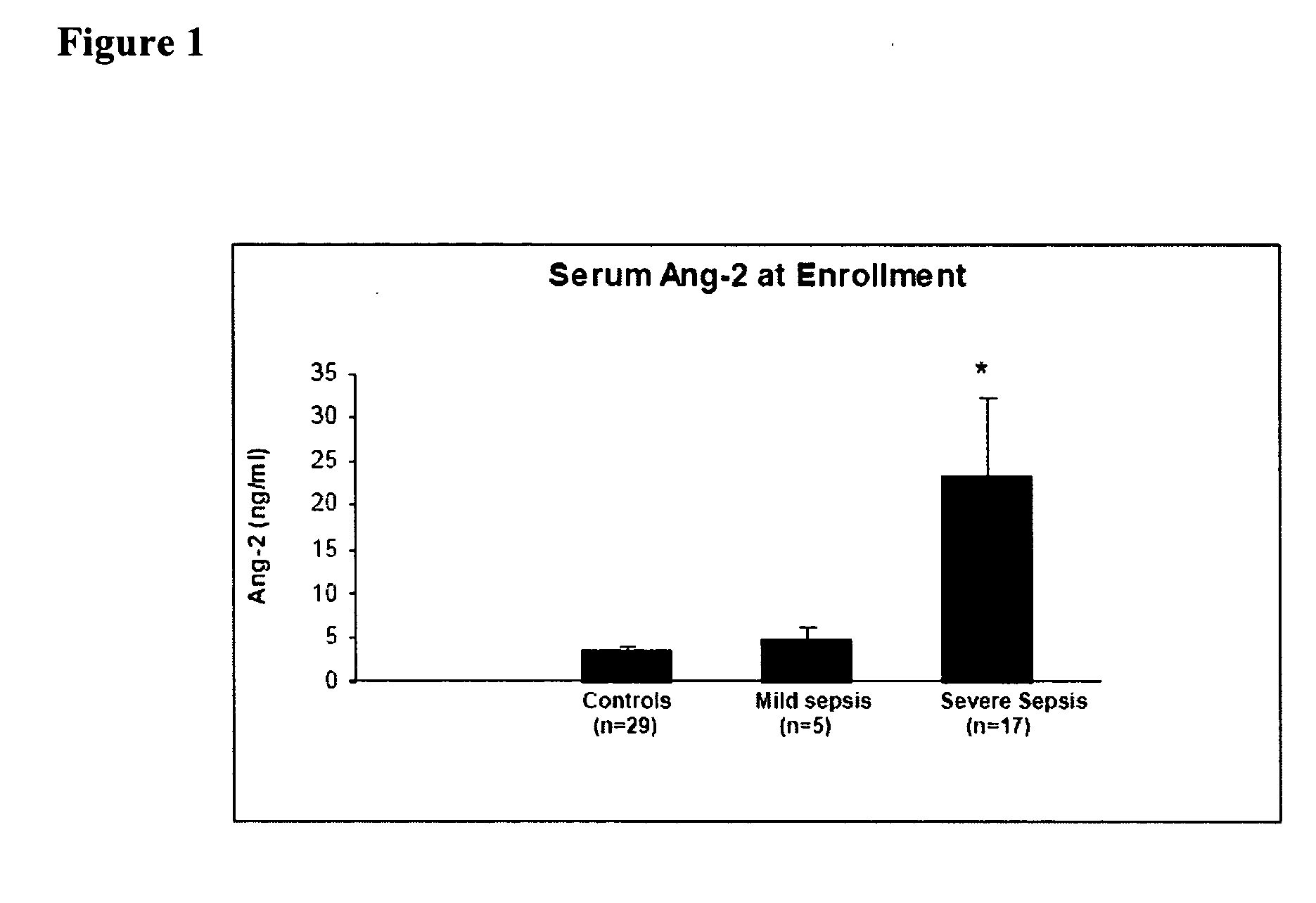
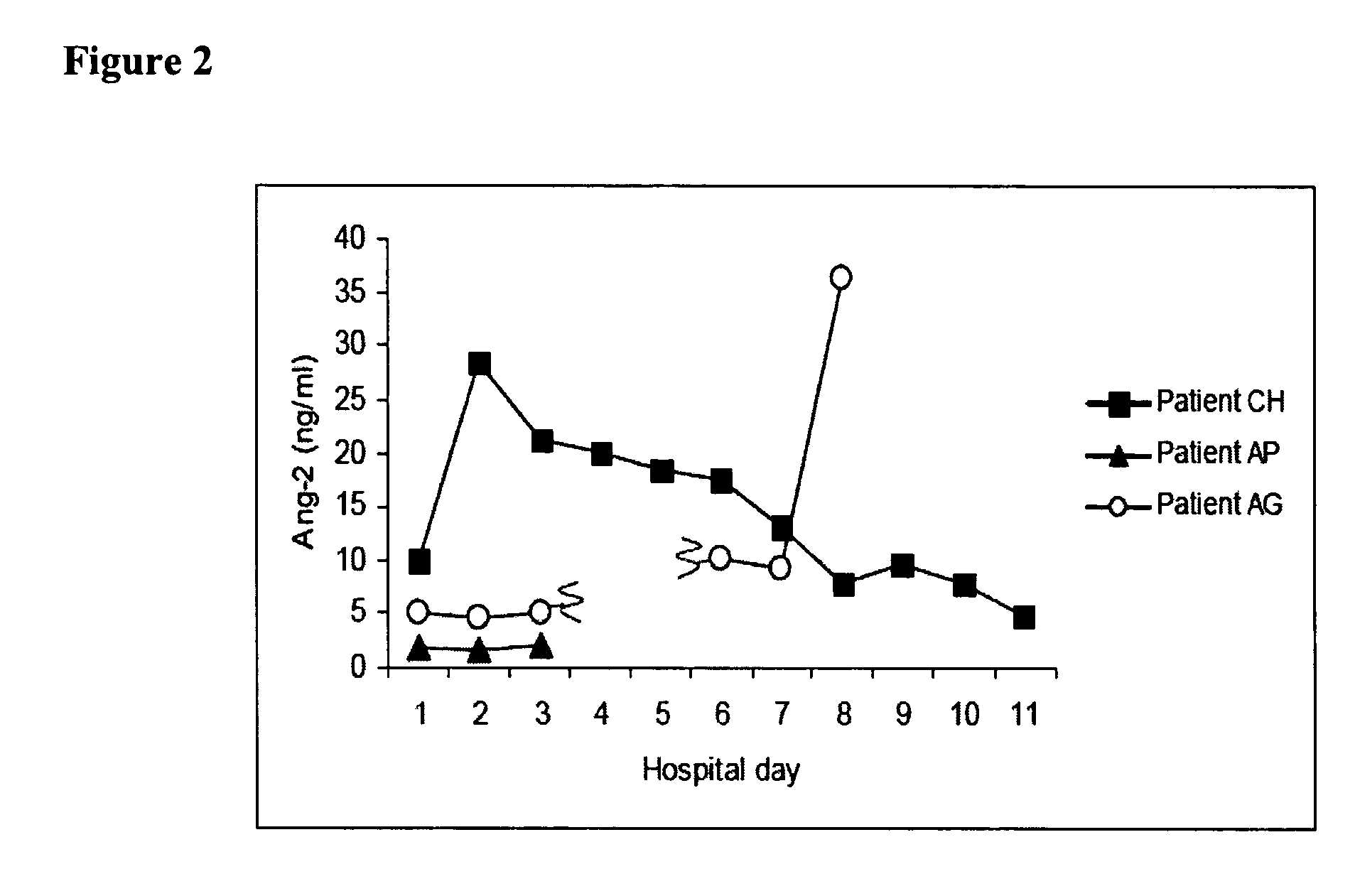
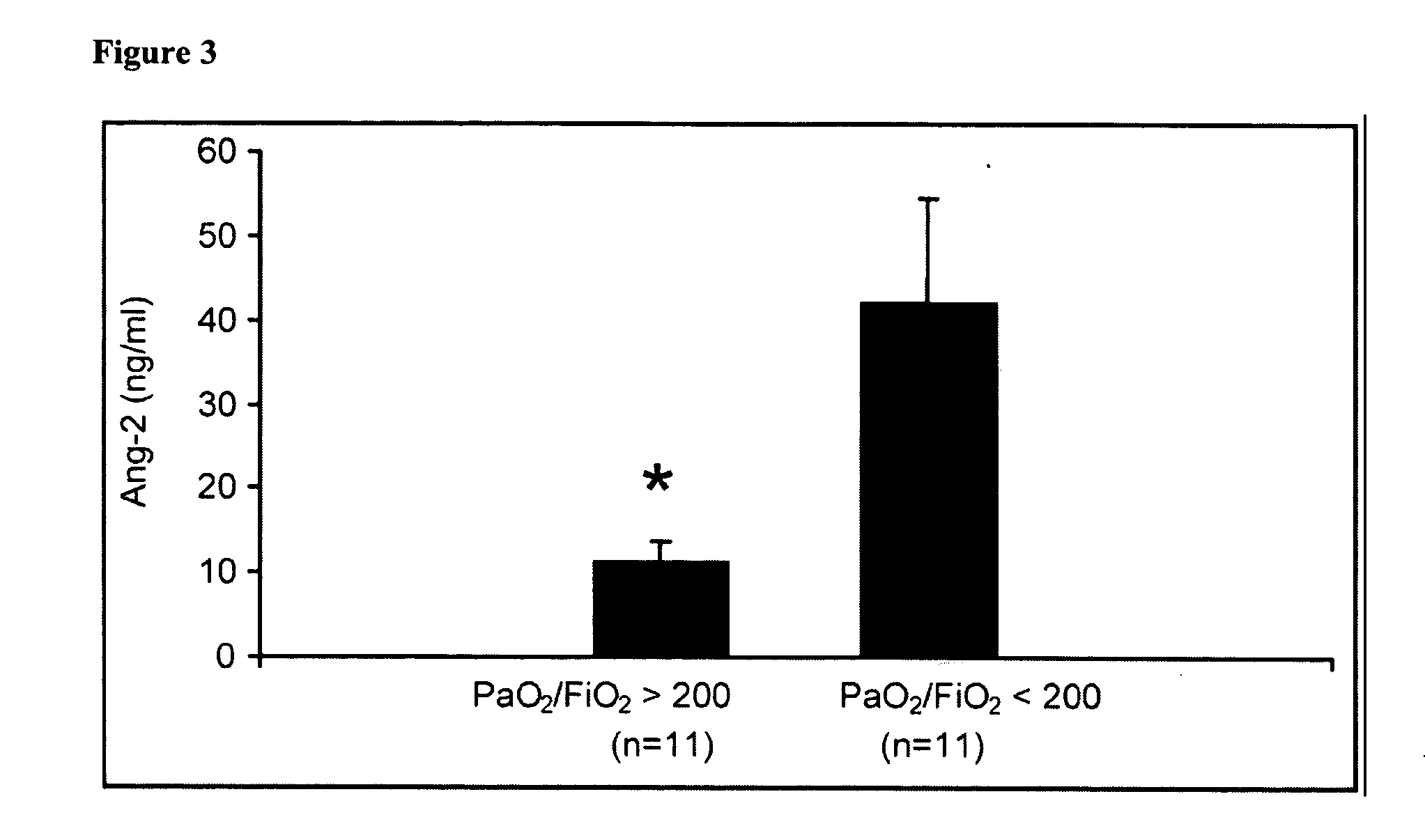
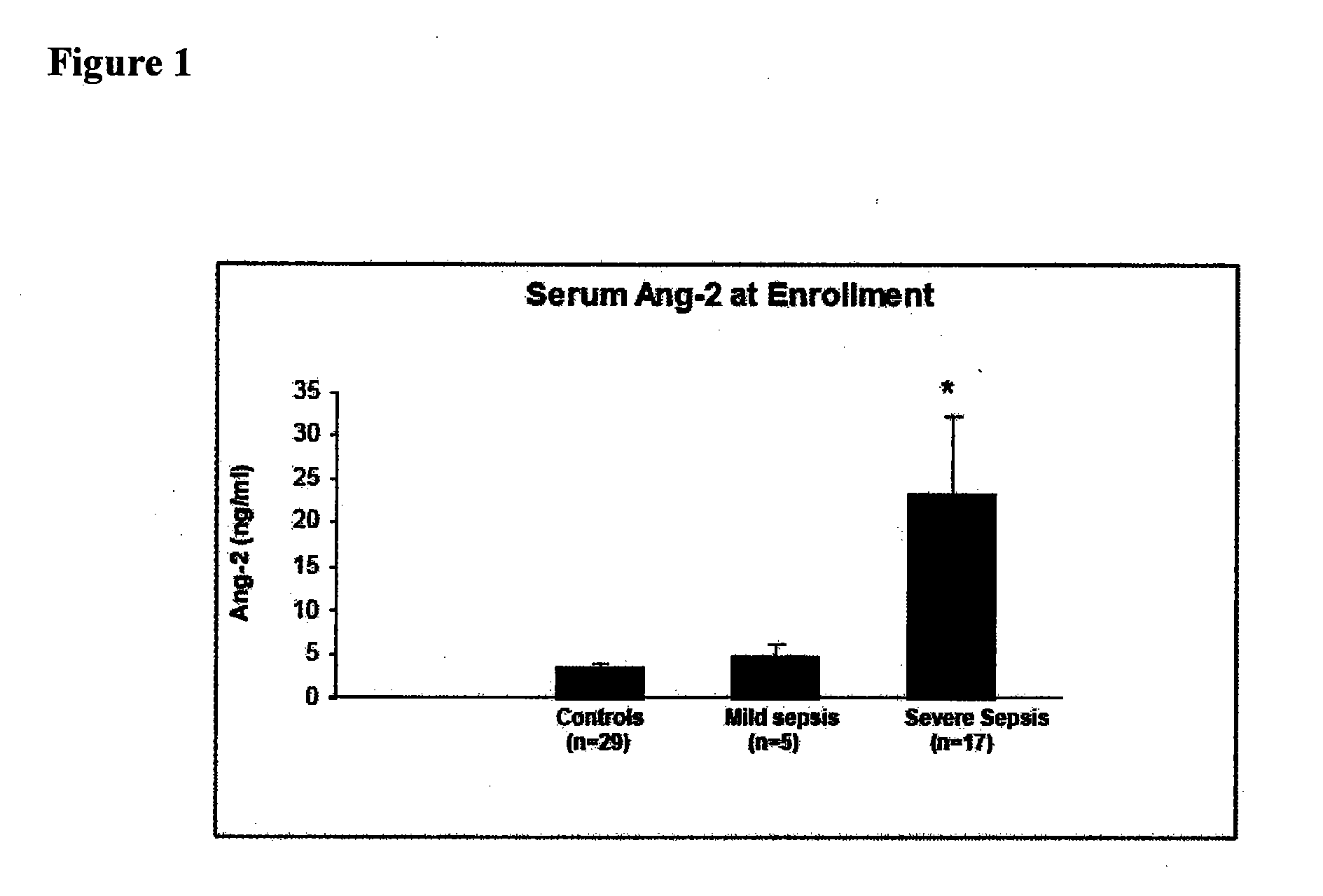
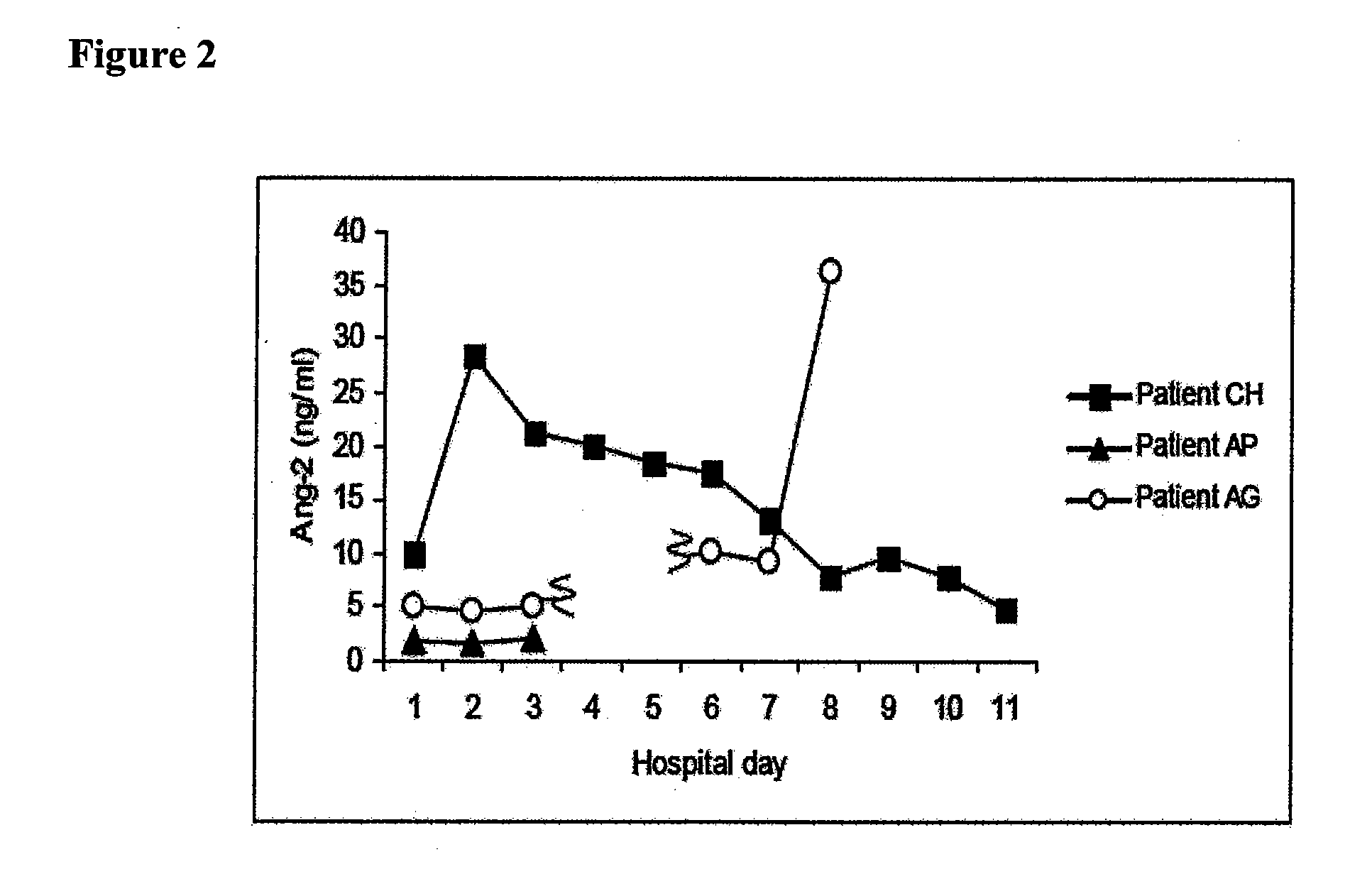
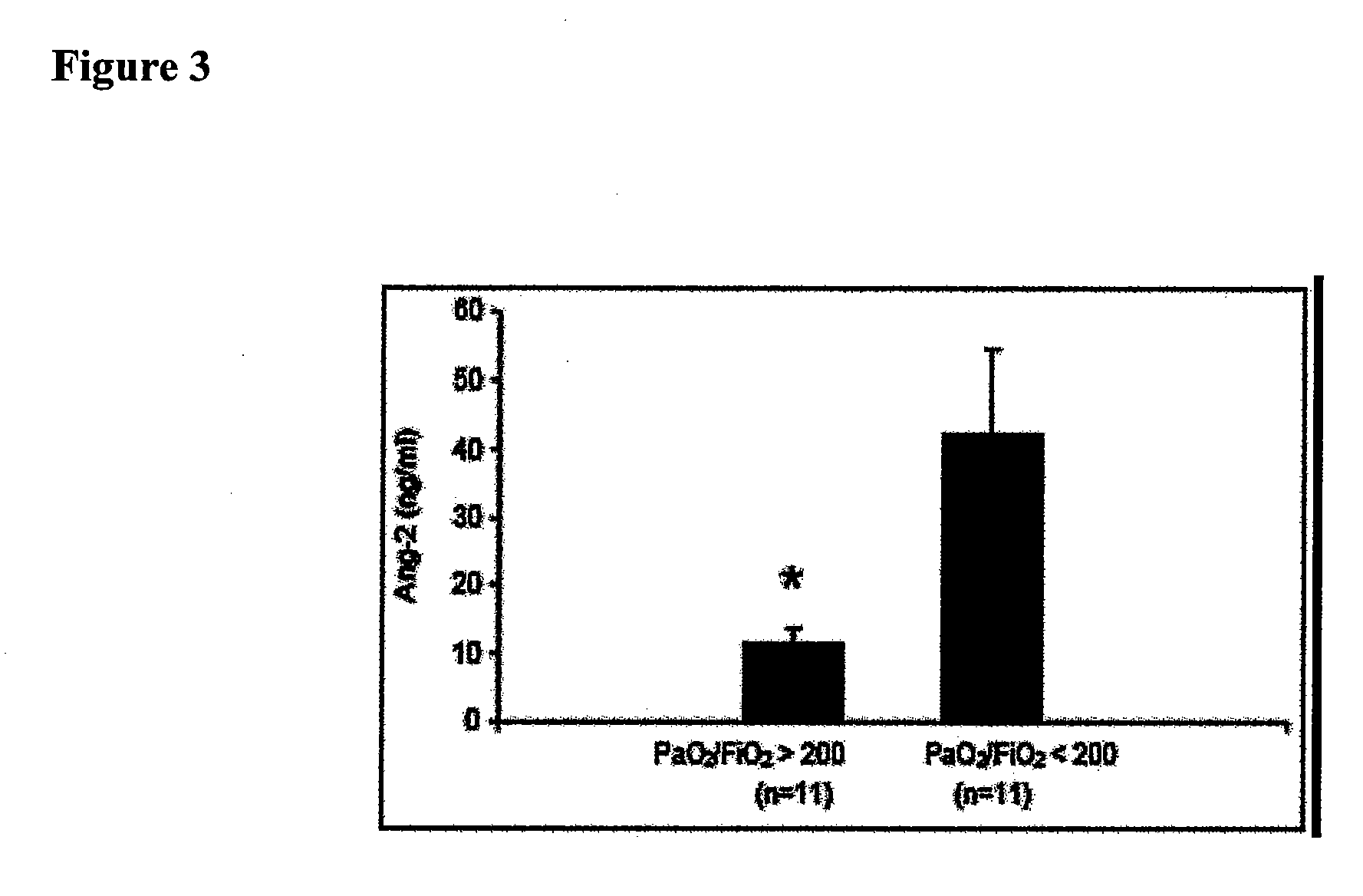
Disclosed herein are methods for treating a vascular leak disorder, hypotension, or a procoagulant state using angiopoietin-2 (Ang-2) antagonist compounds. Also disclosed are methods for treating a vascular leak disorder associated with high dose IL-2 therapy using angiopoietin-2 antagonist compounds. Methods for diagnosing and monitoring vascular leak disorders, hypotension, or a procoagulant state that include the measurement of Ang-2 polypeptide or nucleic acid levels are also disclosed. Methods for inducing a vascular leak using an Ang-2 agonist are also disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
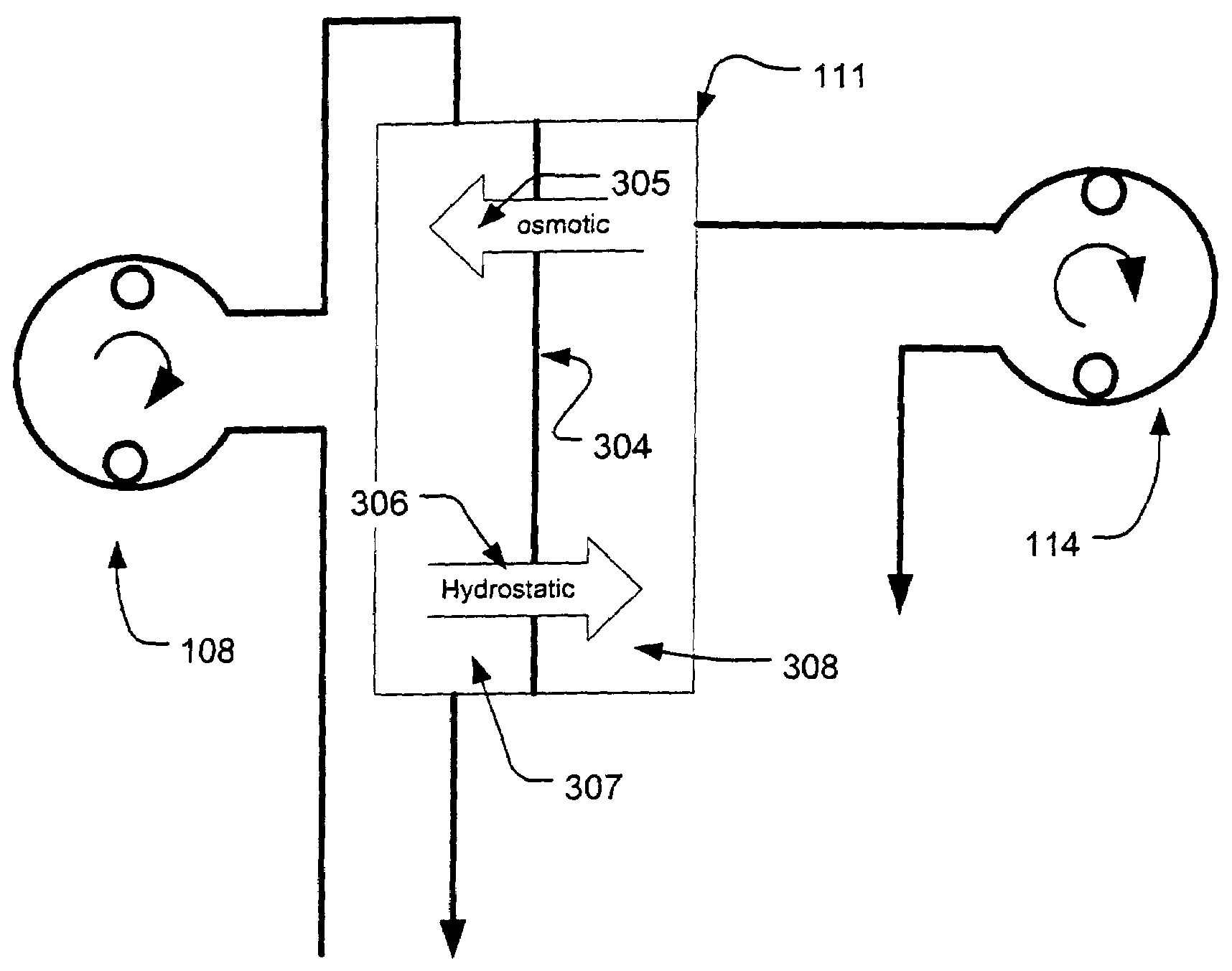
Controller for ultrafiltration blood circuit which prevents hypotension by monitoring osmotic pressure in blood
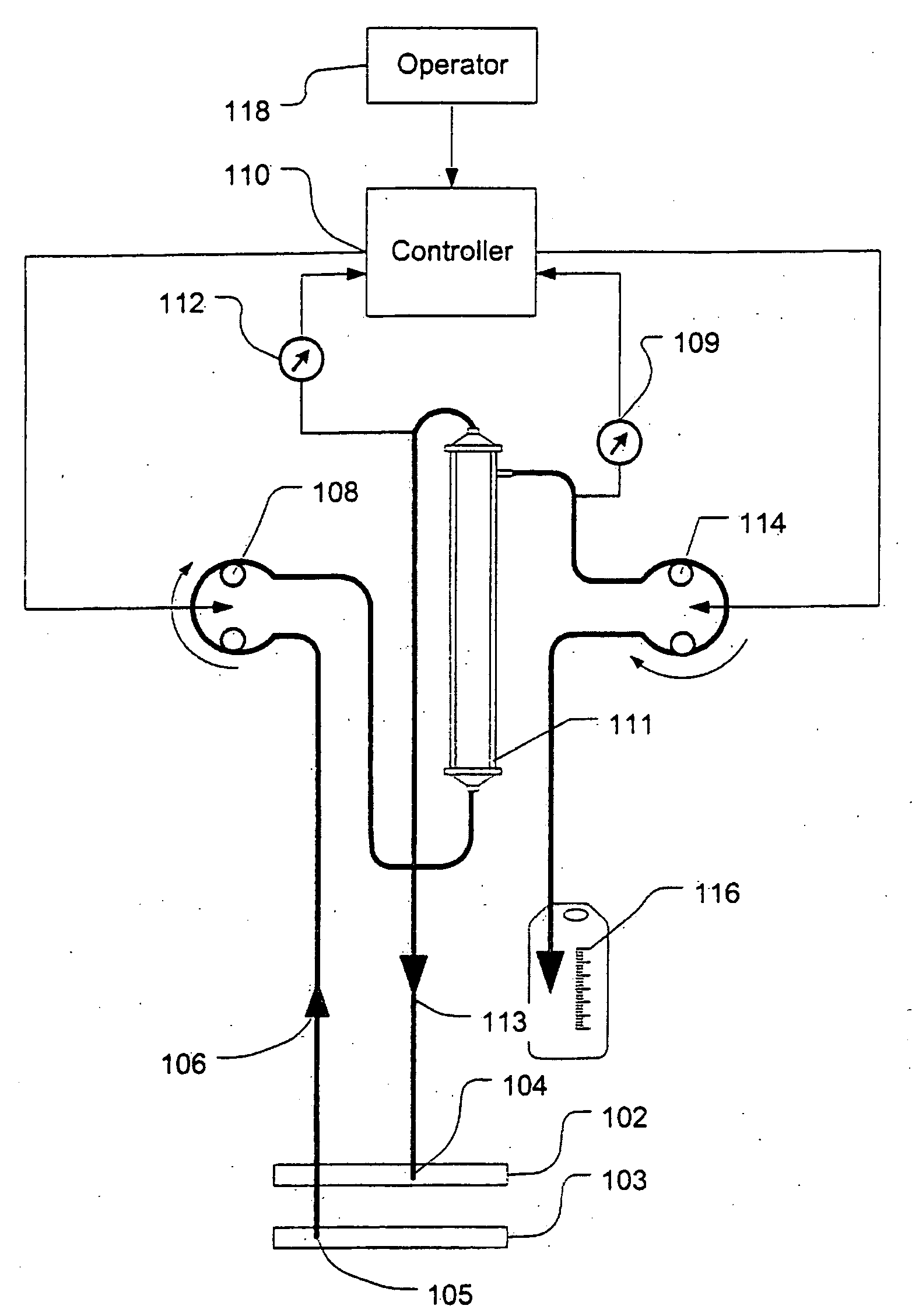
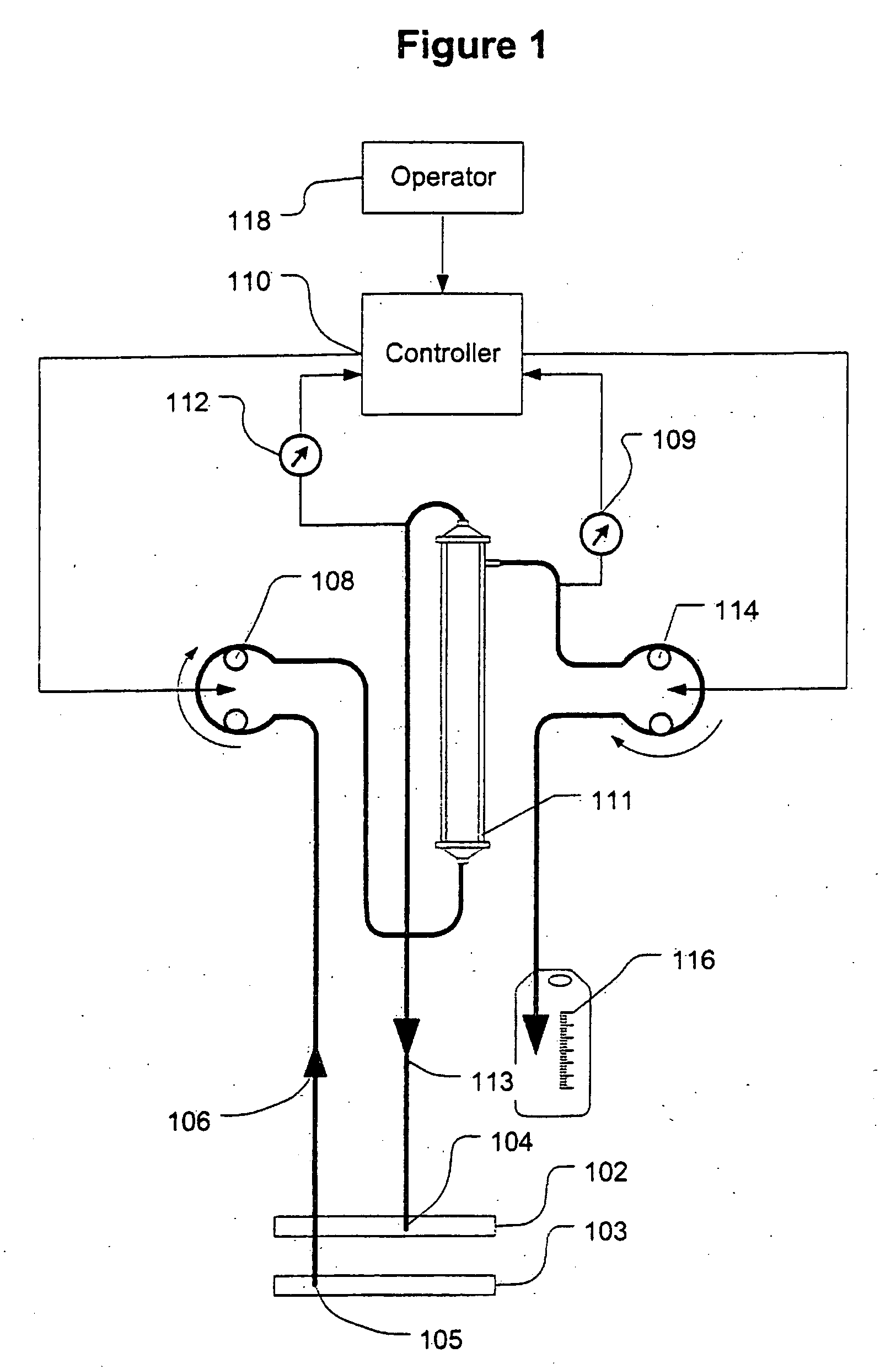
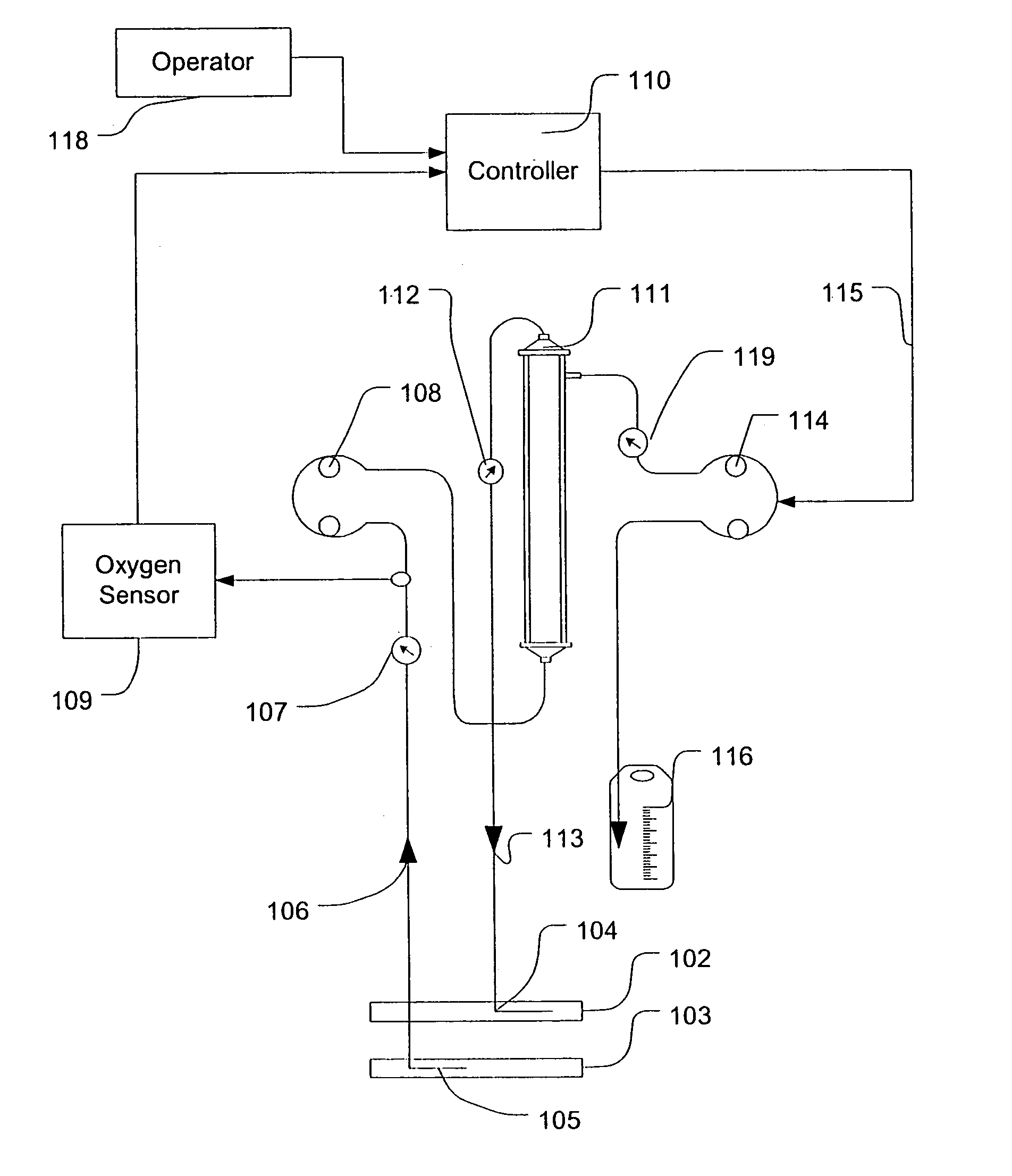
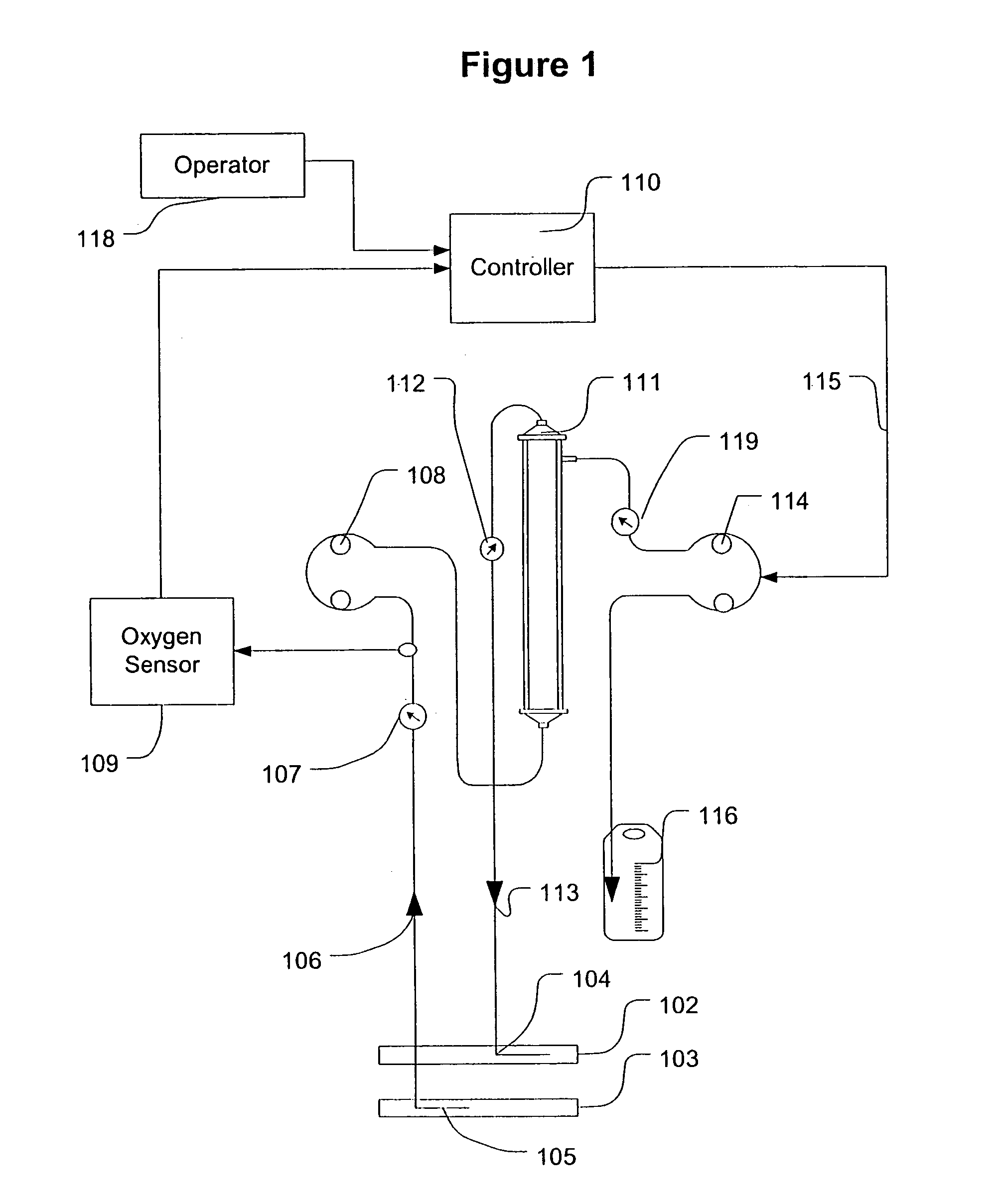
A device for continuously measuring osmotic pressure of blood flowing through an extracorporeal blood circuit including: a blood passage further comprising a withdrawal blood passage connectable to a blood vessel in a patient and an infusion blood passage connectable to a blood vessel in a patient; a filter further comprising a filtrate chamber, a blood chamber and a permeable membrane separating the filtrate chamber and blood chamber, wherein the blood chamber is in fluid communication with the blood passage; a pressure sensor measuring a pressure difference between the filtrate chambers and the blood chamber, and a controller receiving a pressure signal from the pressure sensor, determining an osmotic pressure across the permeable membrane of the filter, and adjusting a rate of removal of fluid from blood in the filter if the determined osmotic pressure level varies from a predetermined osmotic pressure setting.
Owner:GAMBRO LUNDIA AB
Estimation of propensity to symptomatic hypotension
ActiveUS20100094158A1Rapid blood pressureHaemofiltrationDialysis systemsBlood treatmentsThoracic region
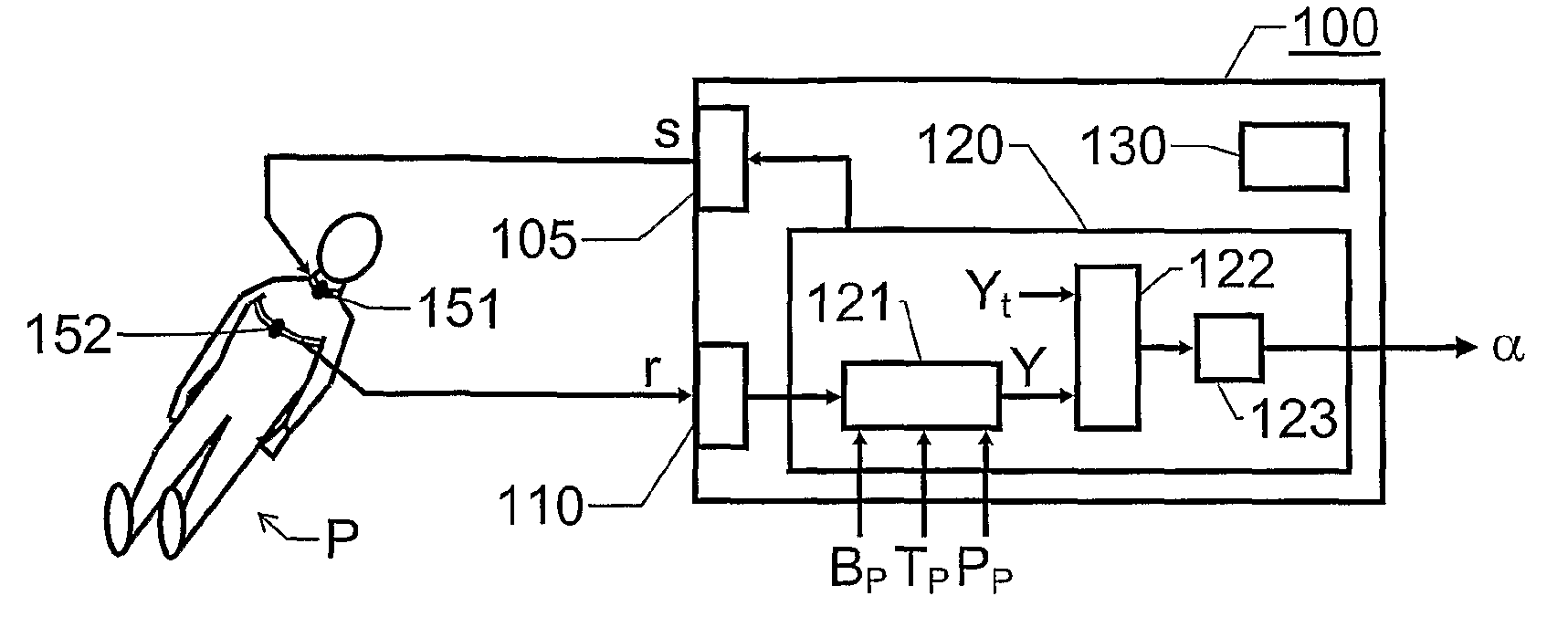
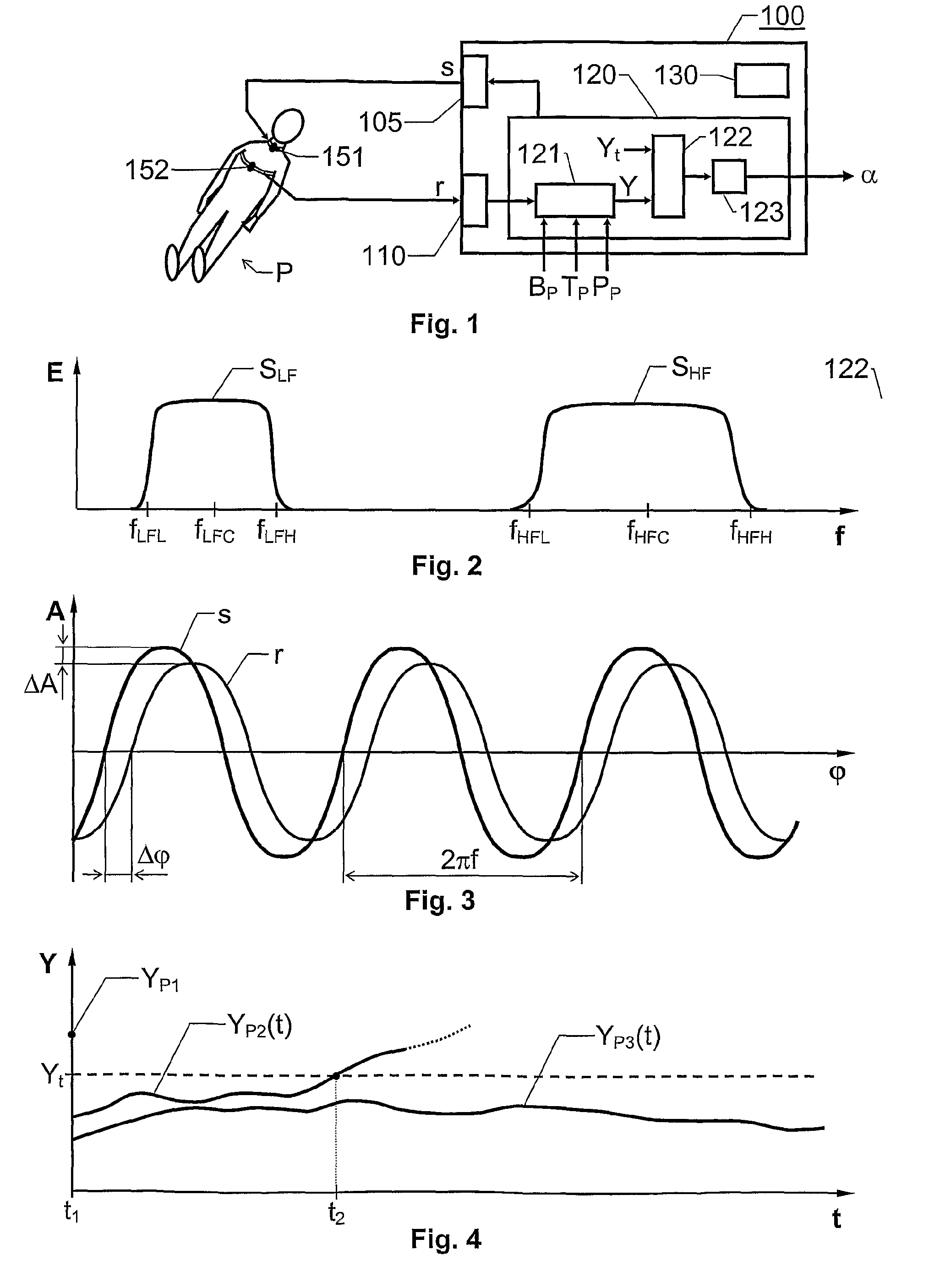
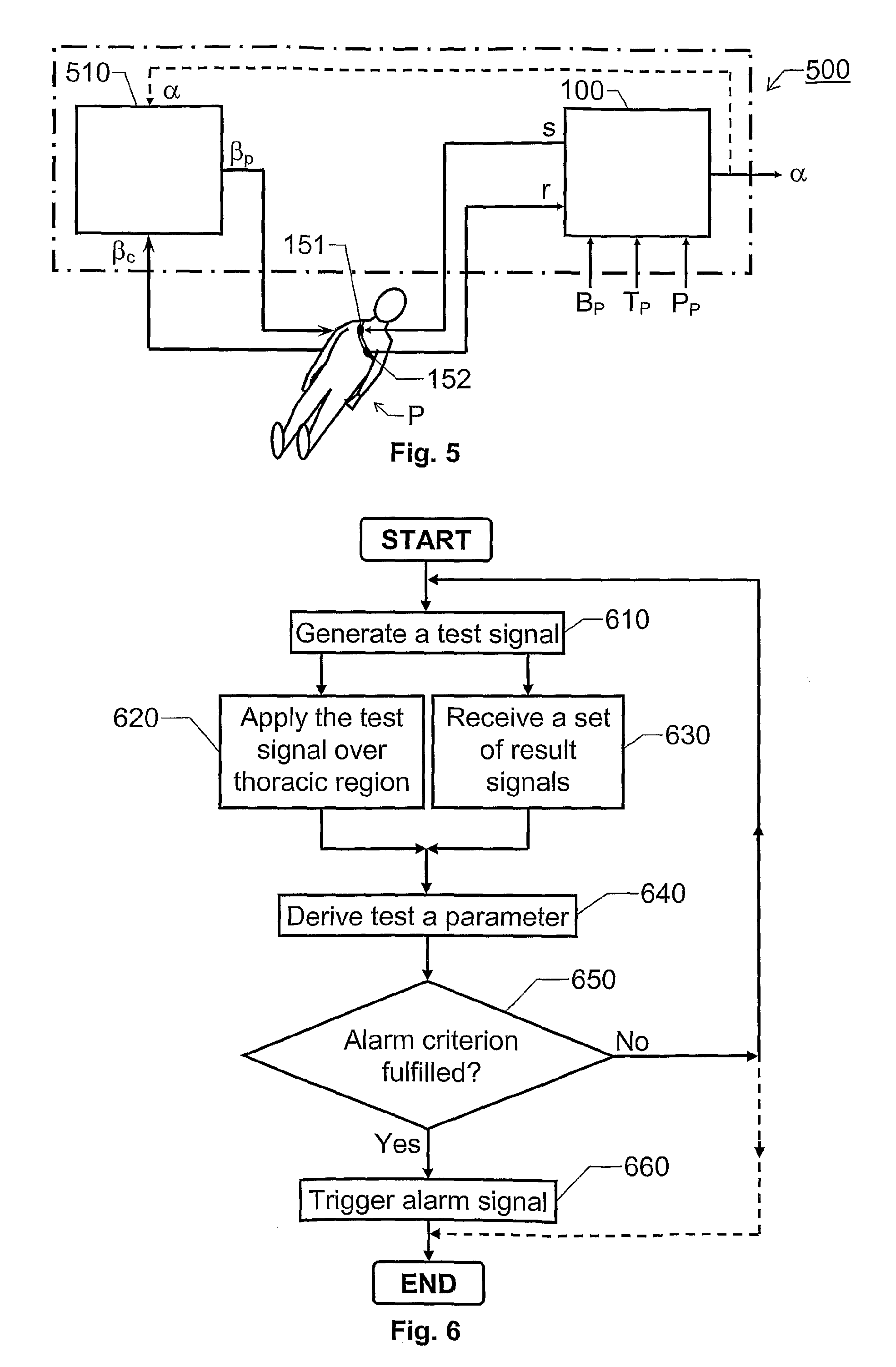
The invention relates to estimation of a patient's propensity to suffer from symptomatic hypotension during extracorporeal blood treatment. An electromagnetic test signal, which is applied over a thoracic region of the patient via at least one transmitter electrode. A result signal produced in response to the test signal is received via at least one receiver electrode on the patient. A test parameter is derived based on the result signal. The test parameter expresses a fluid status of the thoracic region of the patient, and it is determined whether the test parameter fulfills an alarm criterion. If the test parameter fulfills an alarm criteria, an alarm signal is generated. This signal indicates that the patient is hypotension prone, and that appropriate measures should be taken.
Owner:GAMBRO LUNDIA AB
Feedback control of ultrafiltration to prevent hypotension
InactiveUS7175809B2Easy to adaptAvoid hypotensionSemi-permeable membranesOther blood circulation devicesBlood levelVein
A method and system for the extracorporeal treatment of blood to remove fluid from the fluid overloaded patient is disclosed that non-invasively measures an oxygen level in the venous blood. The oxygen blood level is used to detect when hypotension is about to occur in a patient. The oxygen level measurements are used as feedback signals. These feedback signals are applied to automatically control the rate of fluid extraction to achieve the desired clinical outcome and avoid precipitating a hypotensive crisis in the patient.
Owner:GAMBRO LUNDIA AB
Sustained release pharmaceutical composition
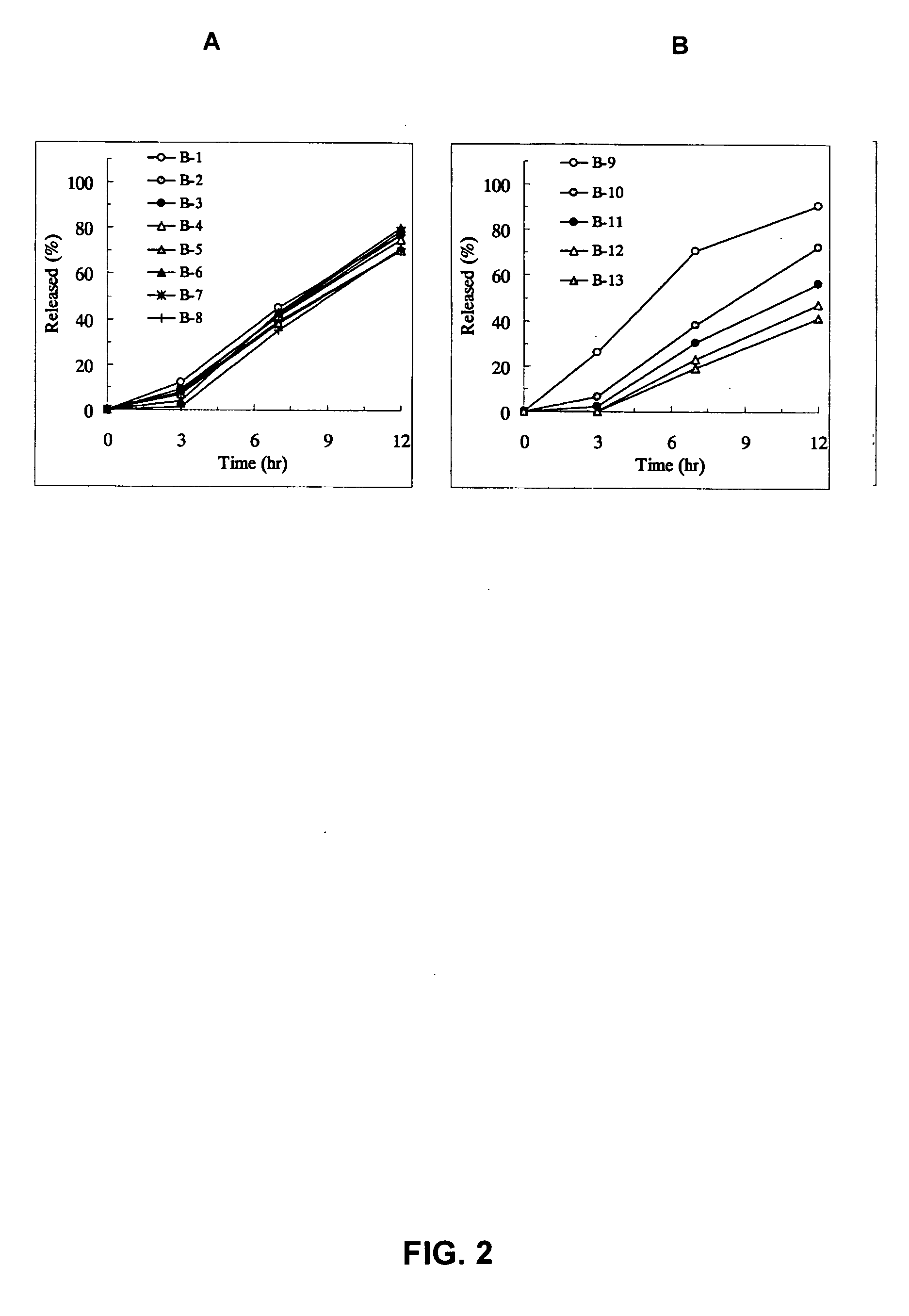
InactiveUS20050100603A1Equivalent and even more efficacyReduce the adverse eventsPowder deliveryBiocideTamsulosin hclSustained release drug
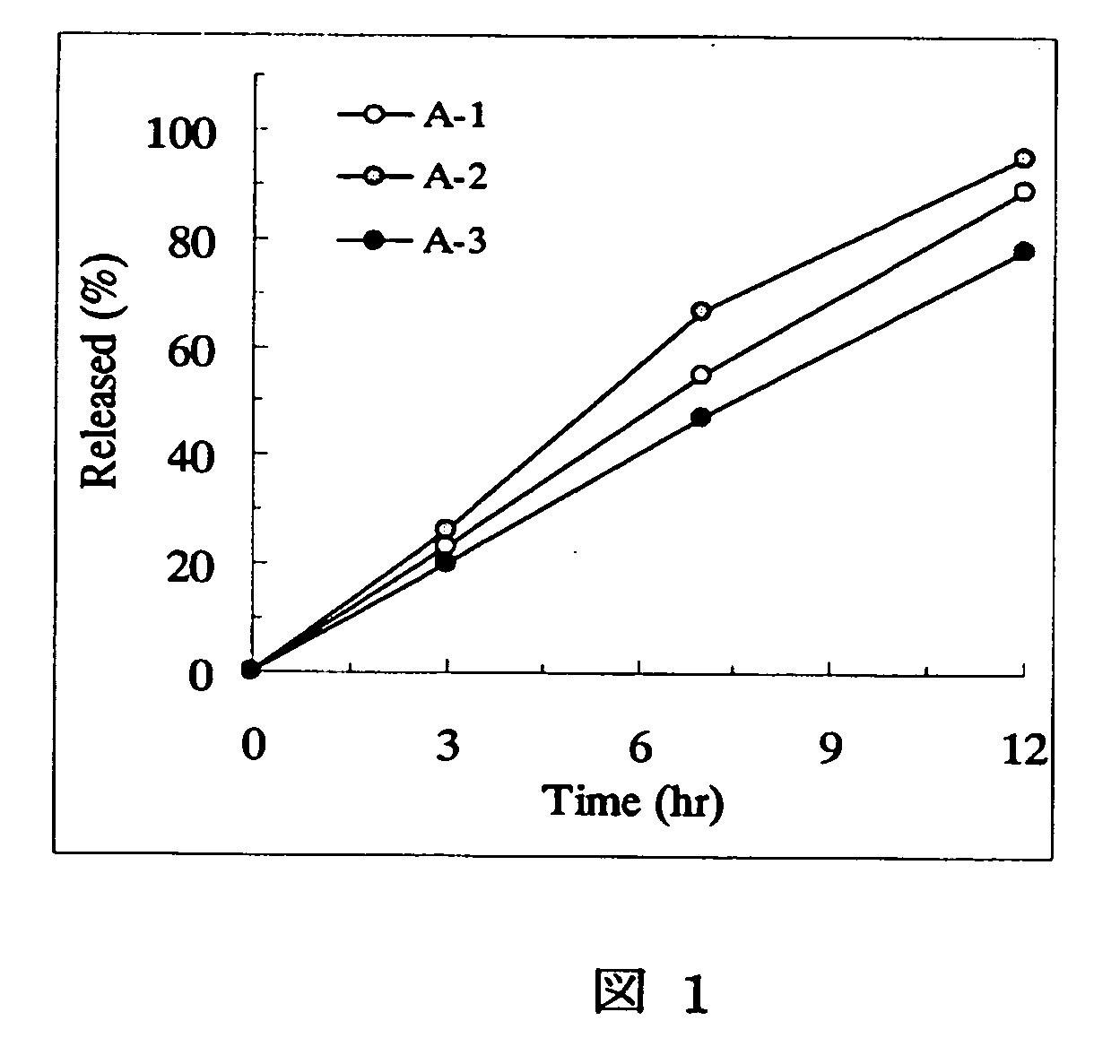
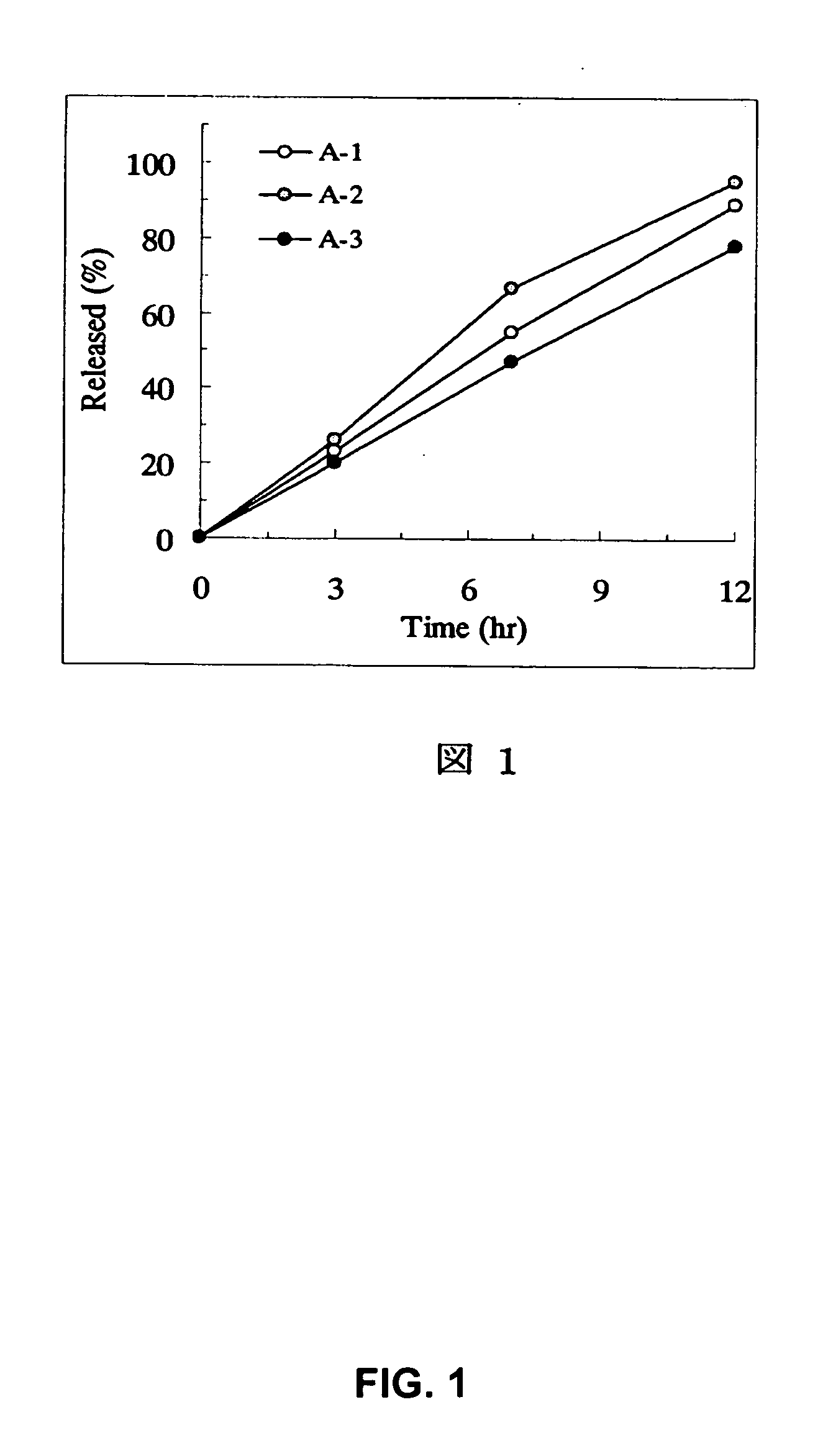
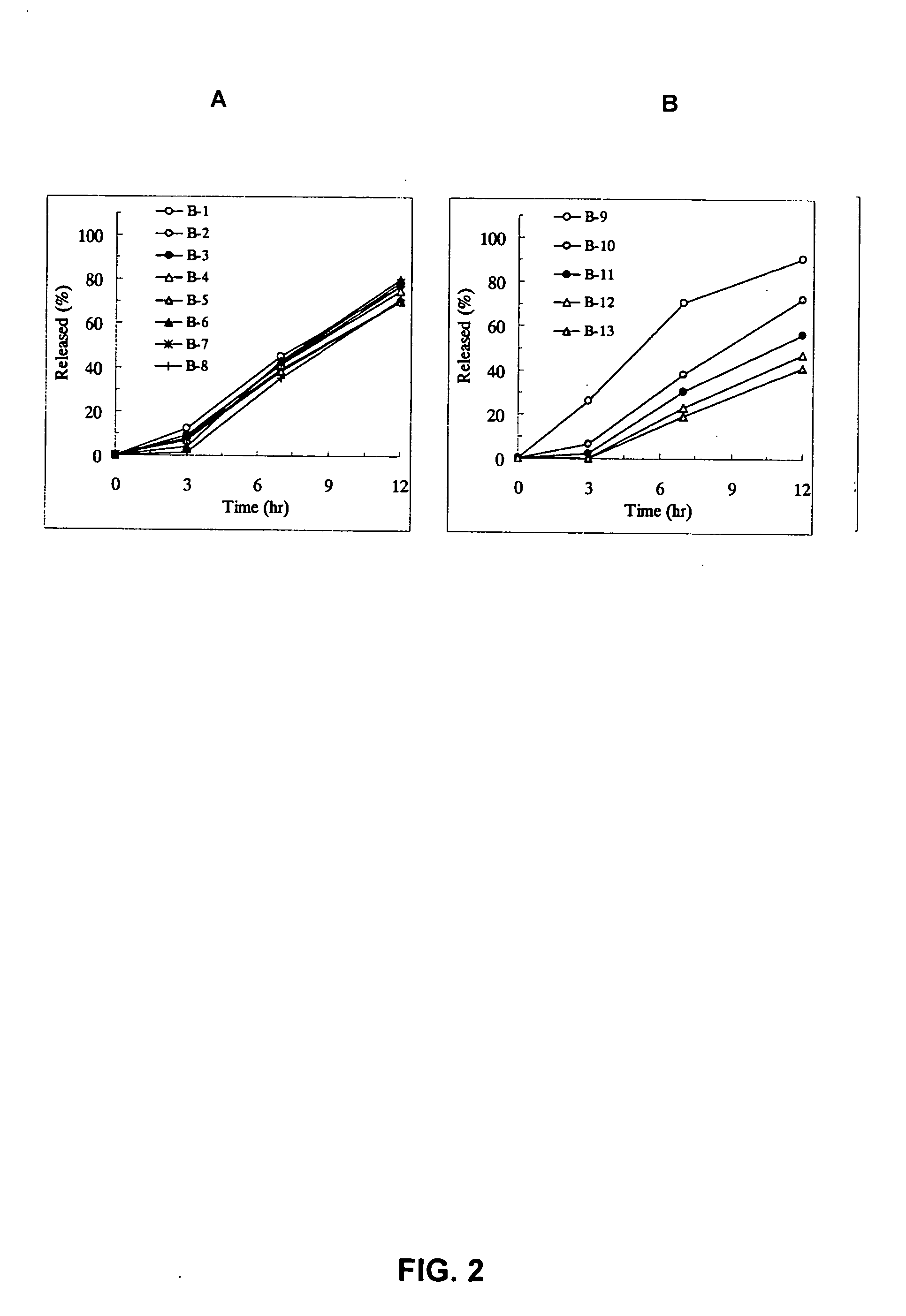
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting at present, it is needed to provide a sustained-release pharmaceutical composition in which the efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited in the dosage and it is also needed to provide a method for administration of tamsulosin hydrochloride in which the adverse reactions accompanied by therapy or prevention on the basis of an α1 receptor blocking action are reduced. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and the ratio (Cmin / Cmax ratio) of the plasma tamsulosin concentration at 24 hours after the administration of the preparation per os (Cmin) to the maximum plasma tamsulosin concentration after the administration (Cmax) is about 0.4 or more.
Owner:ASTELLAS PHARMA INC
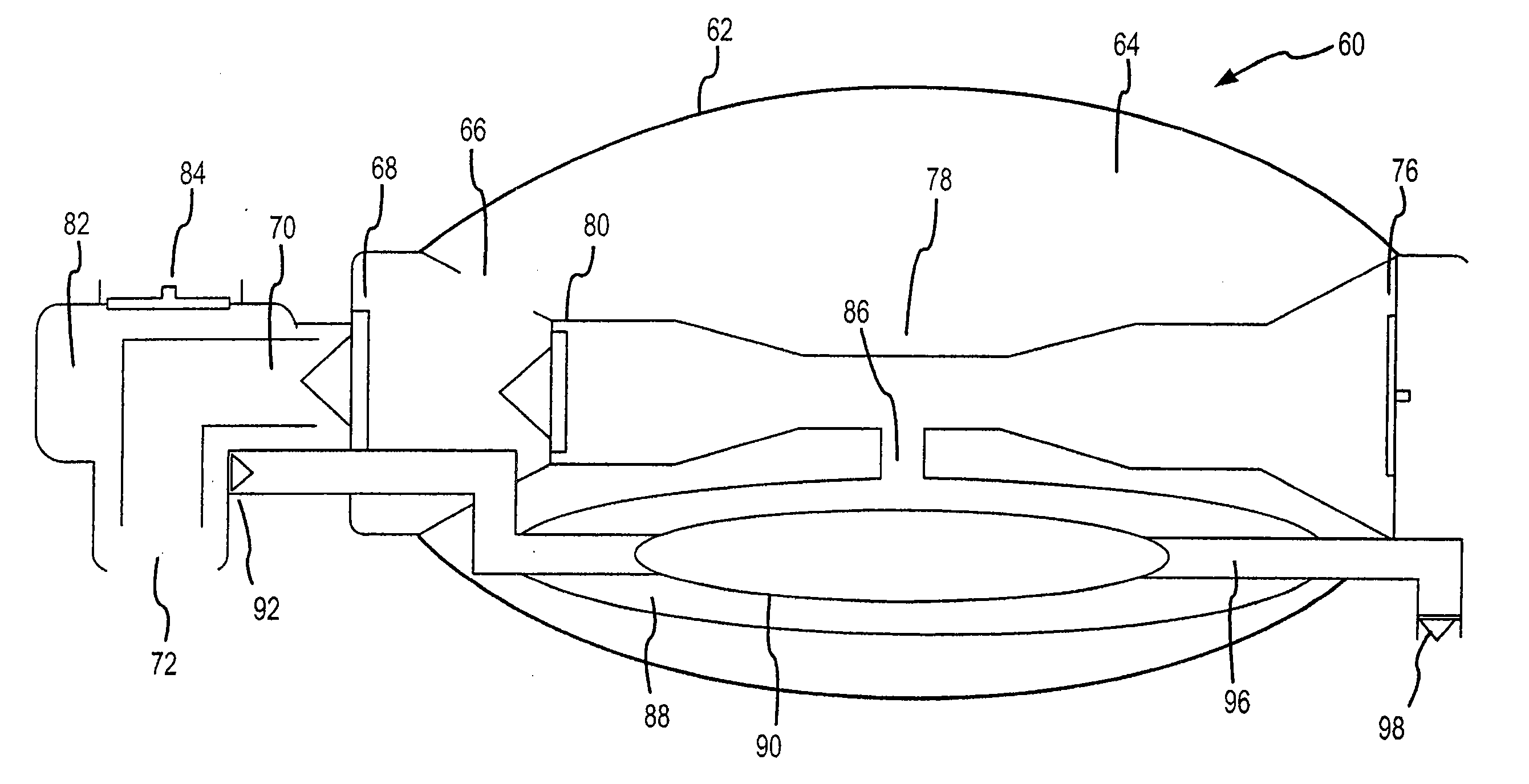
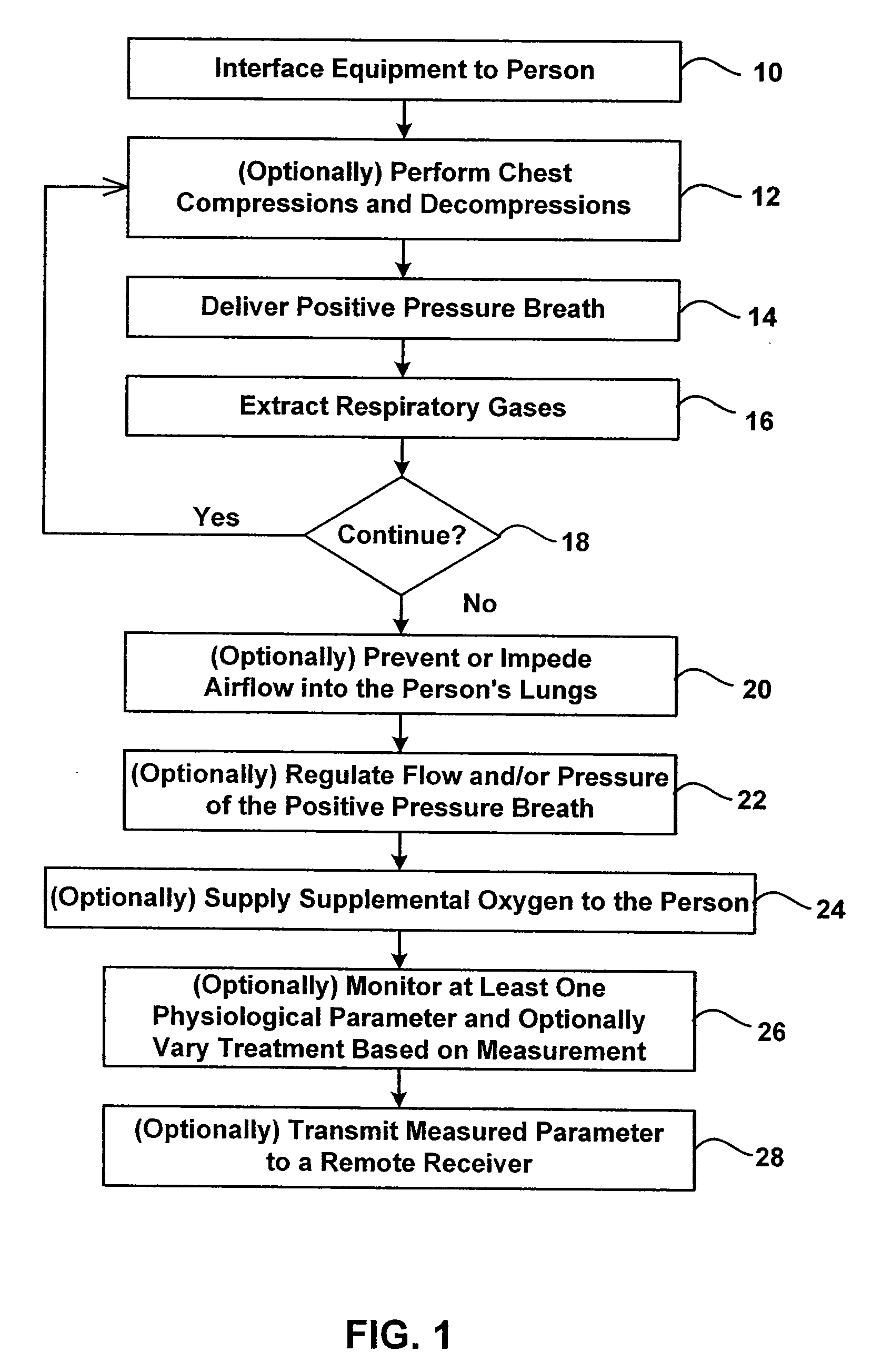
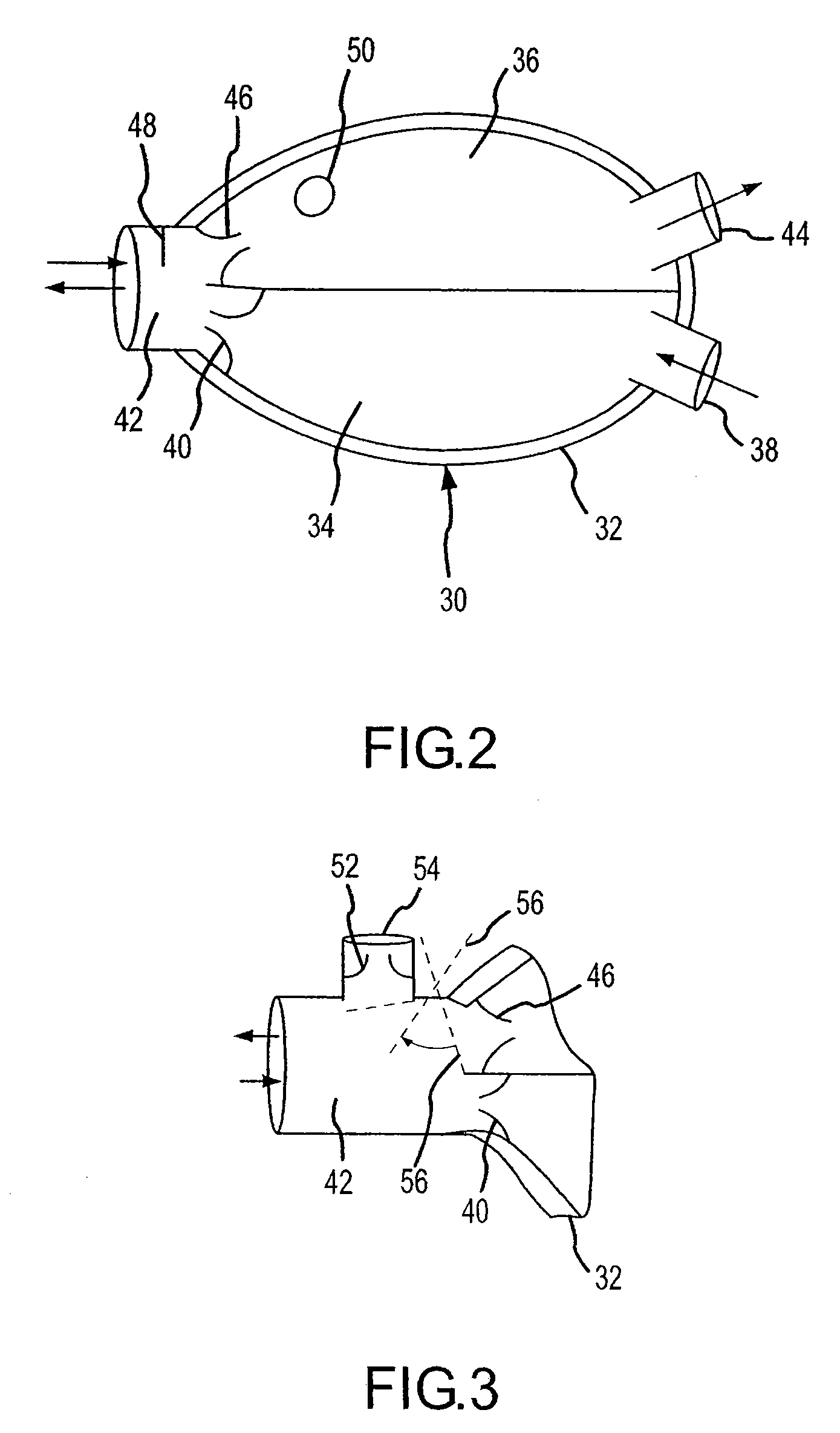
Bag-valve resuscitation for treating of hypotension, head trauma, and cardiac arrest
InactiveUS20080047555A1Enhance venous returnPrevented and impededTracheal tubesOperating means/releasing devices for valvesCardiorespiratory arrestBreathing gas
A device for manipulating intrathoracic pressures comprises a compressible bag structure, and an interface member coupled to the bag structure. A one way forward valve is coupled to the bag structure to permit respiratory gas to flow to the patient when the bag structure is compressed. A one way exit valve is employed to allow respiratory gases to be pulled from the person's airway upon decompression of the bag structure to produce a negative intrathoracic pressure.
Owner:ADVANCED CIRCULATORY SYST
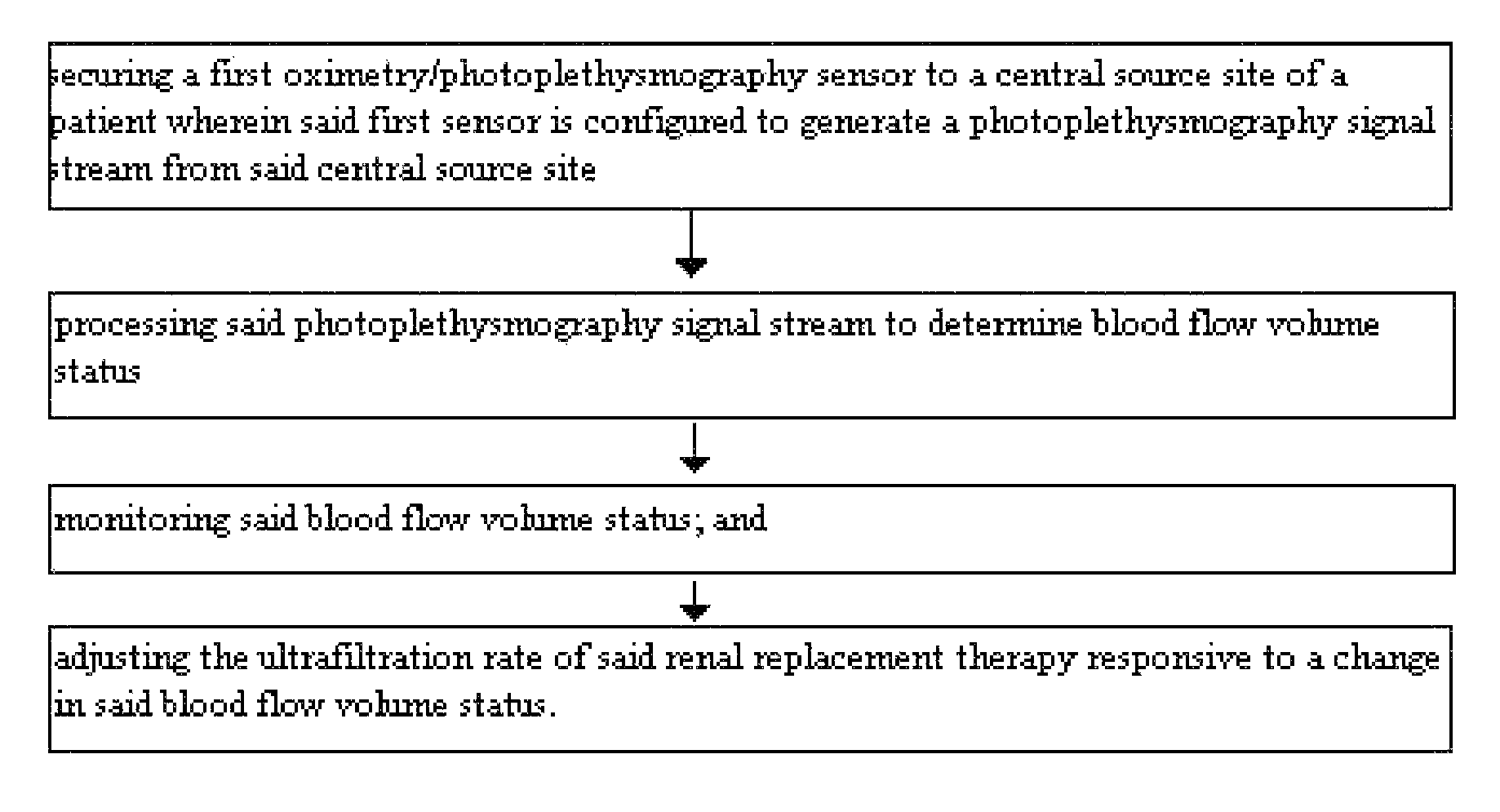
Method for using photoplethysmography to optimize fluid removal during renal replacement therapy by hemodialysis or hemofiltration
Disclosed herein are methods, systems and devices to monitor vascular volume status during renal replacement therapy utilizing at least one oximetry / photoplethysmography sensor. The methods, systems and devices provide an alternative to conventional vascular volume monitoring methods during renal replacement therapy while enabling reliable, non-invasive, and automatic monitoring of vascular volume to avert patient hypotension. The methods, systems and devices may be employed in the context of both inpatient and outpatient dialysis facilities and may also be incorporated into conventional hemodialysis and hemofiltration techniques and equipment.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
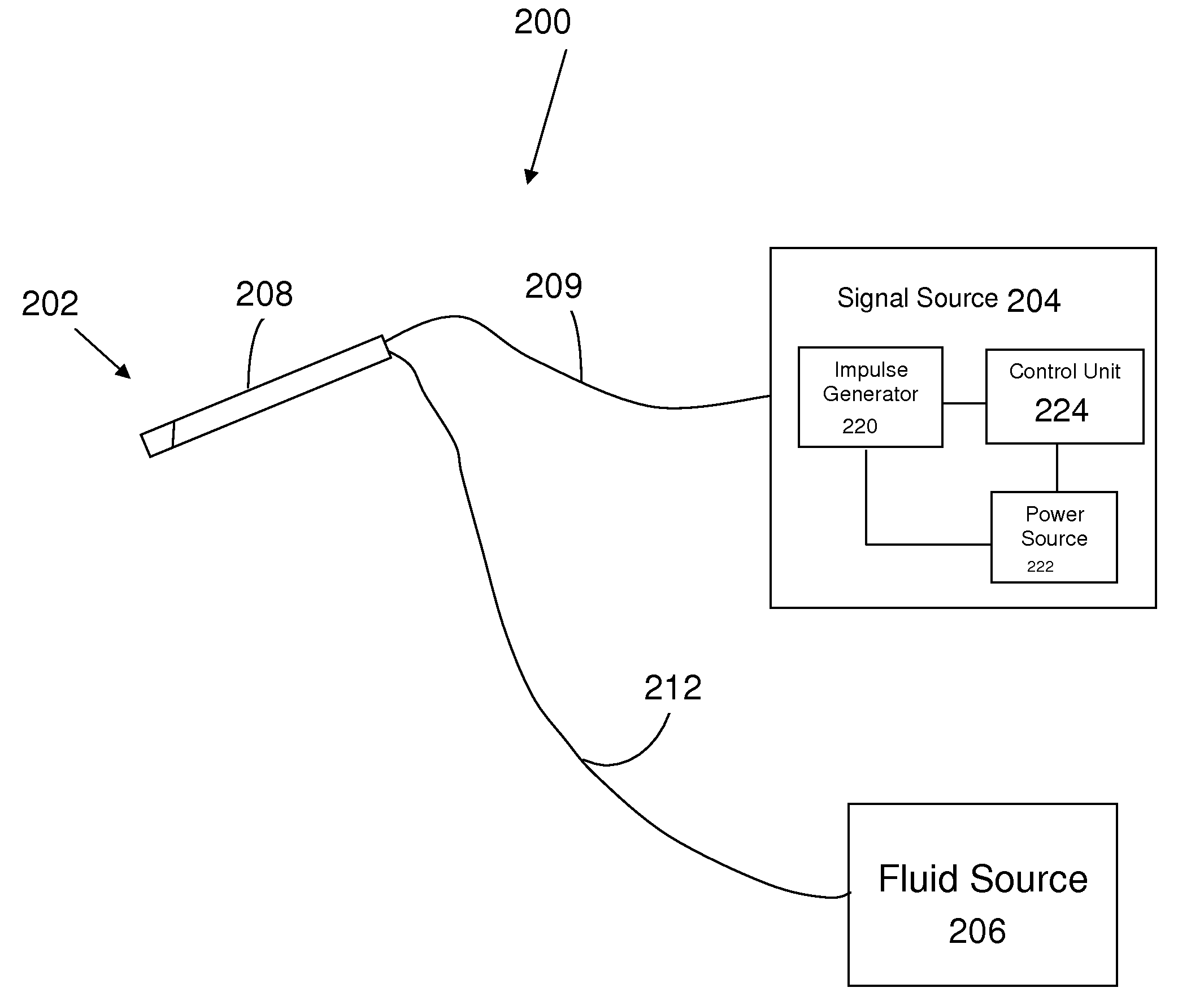
Electrical stimulation treatment of hypotension
ActiveUS8041428B2Raise the ratioSlow down heart rateEsophageal electrodesEndotracheal electrodesNervous systemMedicine
The present invention includes methods and devices for treating hypotension, such as in cases of shock, including septic shock, anaphylactic shock and hypovolemia. The method includes the step of applying at least one electrical impulse to at least one selected region of a parasympathetic nervous system of the patient. The electrical impulse is sufficient to modulate one or more nerves of the parasympathetic nervous system to increase the ratio of blood pressure to heart rate and relieve the condition and / or extend the patient's life.
Owner:ELECTROCORE
Co-Administration of an Agent Linked to an Internalization Peptide with an Anti-Inflammatory
ActiveUS20090176713A1Reduce capacityInhibit the inflammatory responseOrganic active ingredientsNervous disorderCo administrationBiotin
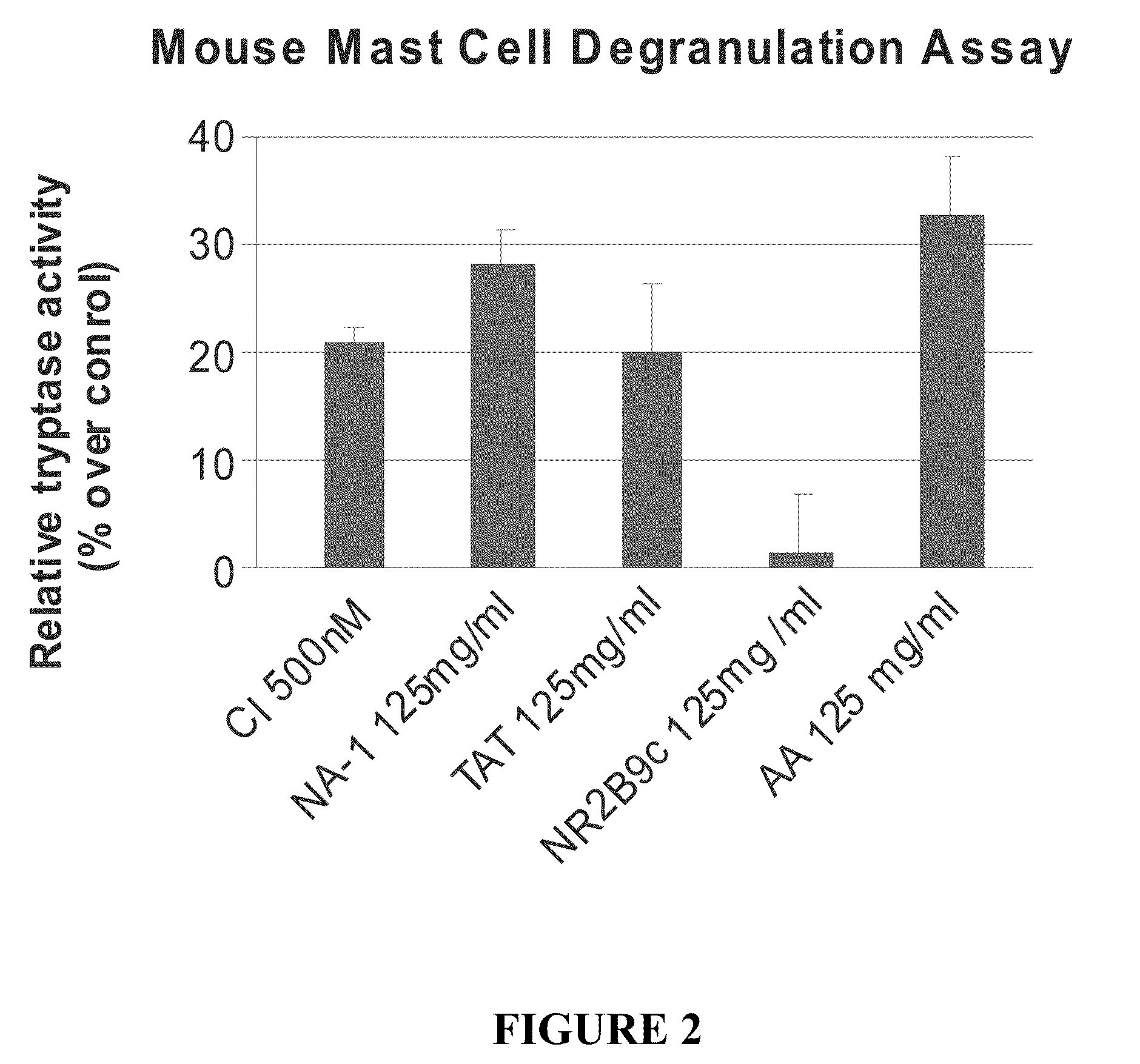
The invention provides methods of delivering pharmacologic agents linked to an internalization peptide, in which an inflammatory response inducible by the internalization peptide is inhibited by co-administration of an anti-inflammatory or by linking the internalization peptide to biotin or similar molecule. Such methods are premised in part on the results described in the examples whereby administration of a pharmacological agent linked to tat high dosages is closely followed by an inflammatory response, which includes mast cell degranulation, histamine release and the typical sequelae of histamine release, such as redness, heat, swelling, and hypotension.
Owner:NONO INC
Prevention of hypotension and stabilization of blood pressure in hemodialysis patients
InactiveUS20090018206A1Preventing hypotensionPrevents hypotensionBiocideAmine active ingredientsHaemodialysis machineBlood pressure
The present invention relates to the use of S-alkylisothiouronium derivatives, including S-ethylisothiouronium diethylphosphate, for stabilizing blood pressure in hemodialysis patients. The compositions of the invention are effective in preventing hypotension in hemodialysis patients.
Owner:MEDITOR PHARMA
Sustained release pharmaceutical composition
InactiveUS20050100602A1Reduces adverse eventImprove featuresPowder deliveryBiocideTamsulosin hclJapanese Pharmacopoeia
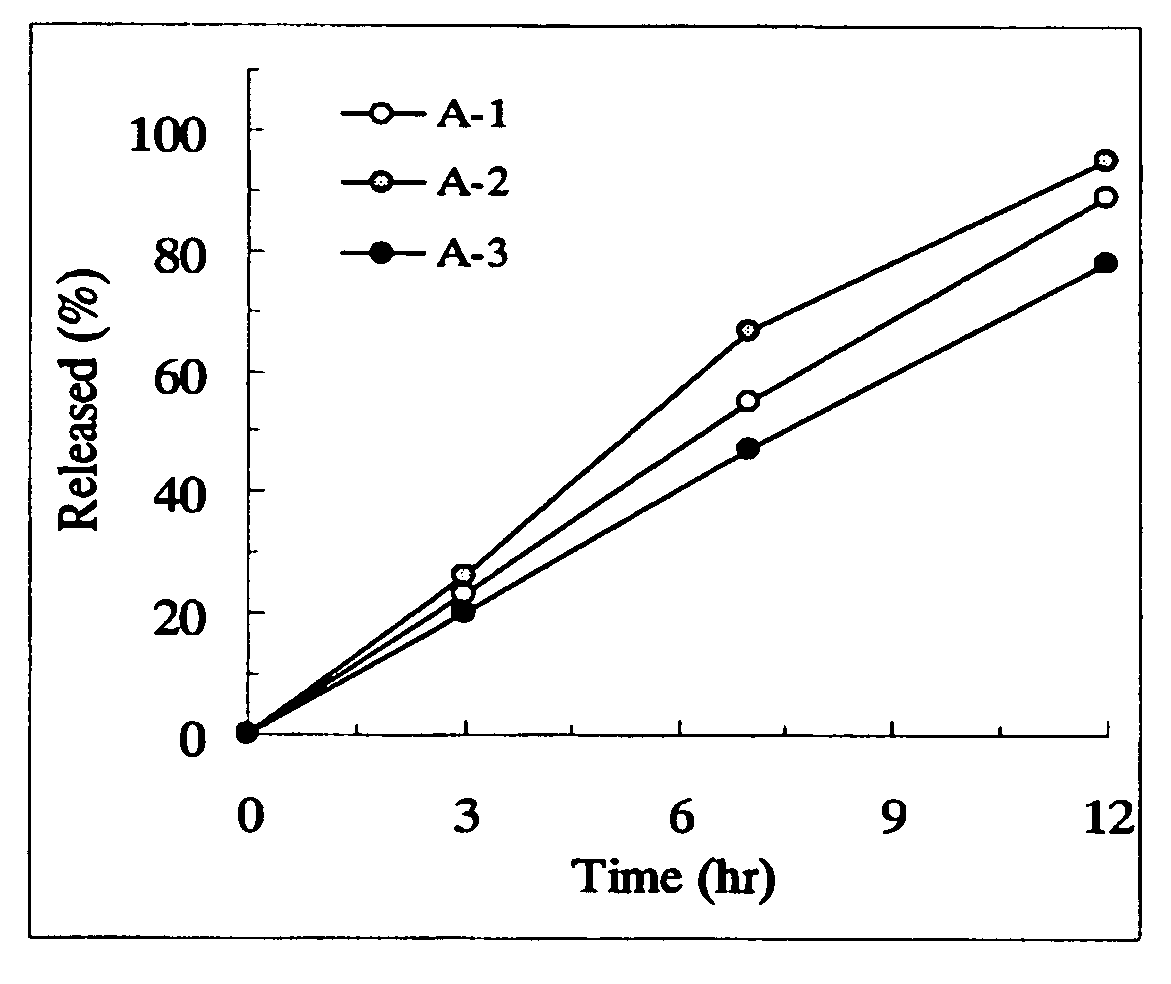
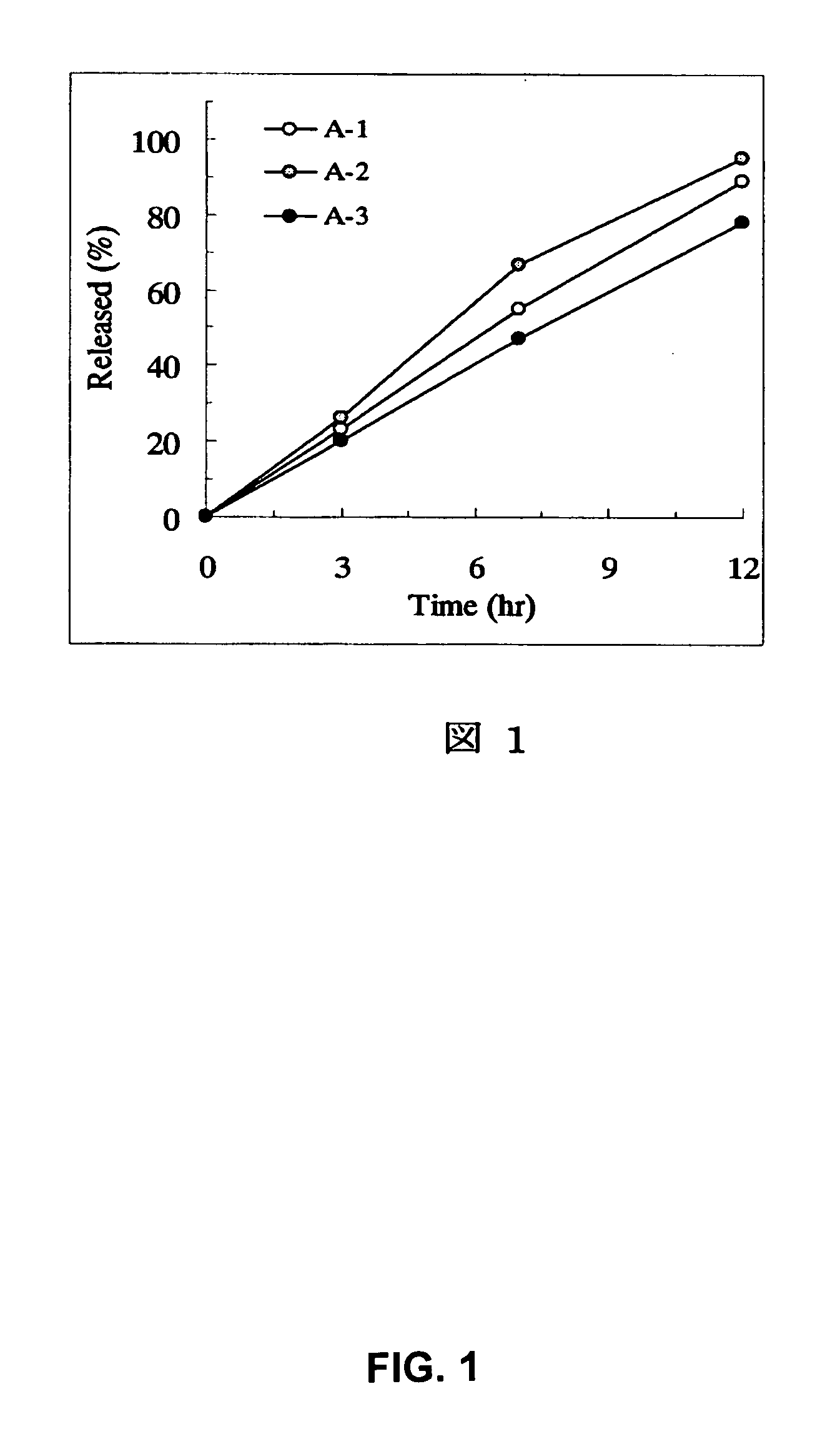
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting, there is a problem to provide a sustained-release pharmaceutical composition in which efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
Traditional Chinese medicine combination of hypotension tranquilization pillow inner
InactiveCN101361920AReasonable and effectiveDredge the meridiansPillowsNervous disorderDiseaseCervical spondylosis
The invention relates to a Chinese medicine composition used in a hypertension-relieving and tranquilizing pillow, which belongs to the field of bed health-care products. The technical proposal thereof is: the composition comprises the raw materials with the following weight portion: 15 portions to 20 portions of Bluish dogbane, 20 portions to 30 portions of Semen Cassiae Torae, 10 portions to 20 portions of Rhizoma Ligustici Wallichii, 10 portions to 20 portions of Tree Peony Bark, 10 portions to 20 portions of Angelica dahurica, 10 portions to 20 portions of asarum, 10 portions to 20 portions of Selfheal, 5 portions to 10 portions of Borneol, 15 portions to 20 portions of Chinese Angelica, 15 portions to 25 portions of Cortex Eucommiae, 10 portions to 20 portions of Honeysuckle, 20 portions to 30 portions of flower of Chinese Scholartree, 20 portions to 40 portions of Mulberry leaf, 50 portions to 80 portions of Silkworm dropping, 15 portions to 25 portions of Gardenia Jasminoides, 10 portions to 20 portions of Rhizoma Gastrodiae, 10 portions to 20 portions of Radix Cyathulae, 10 portions to 20 portions of Radices Paeoniae Alba, 10 portions to 30 portions of Virgate Wormwood Herb and 15 portions to 30 portions of dried Rehmannia root. The formula is reasonable and the effects of activating blood circulation to dissipate blood stasis and dredging channels and collaterals as well as relieving hypertension and tranquilizing are remarkable. In addition, the Chinese medicine composition can prevent dizziness and headache caused by the elevation of blood pressure and diseases that threaten life such as cerebral vascular accident and cerebral thrombosis, and has remarkable effect on preventing cervical spondylosis.
Owner:李富祥
Electrical stimulation treatment of hypotension
Methods and devices for treating hypotension, such as in cases of shock, including septic shock and anaphylactic shock, wherein the treatment includes providing an electrical impulse to a selected region of the vagus nerve of a patient suffering from hypotension to block and / or modulate nerve signals that regulate blood pressure.
Owner:ELECTROCORE
Cassia obtusifolia tea bag with functions of weight reduction, hypotension and hypoglycemic effect and its production process
The invention provides a cassia obtusifolia tea bag with functions of weight reduction, hypotension and hypoglycemic effect, which is characterized in that the production process comprises the following steps: washing, cleaning and drying the cassia obtusifolia stem, leaf and grain, crushing by a pulverizer to 280 meshes, uniformly stirring, packing, disinfecting to obtain the cassia obtusifolia tea bag with functions of lipid lowering, hypotension and hypoglycemic effect. The optimal composition and the weight part ratio of the tea bag are: 40-60 parts of dried cassia obtusifolia stem, leaf and grain, 10 parts of dried lotus leaf, 5 parts of haw, 5 parts of dried balsam pear, 5 parts of Chinese wolfberry, 10 parts of Pu'er tea, 10 parts of cassia seed, 5 parts of mulberry leaf, 5 parts of folium apocyni veneti, 5 parts of Chinese yam as medicine, 3 parts of rhubarb, 2 parts of chrysanthemum, 2 parts of honeysuckle flower, 1 part of boat-fruited sterculia, 5 parts of cassia seed, 5 parts of kudzu root and 10 parts of additive. The additive is composed of fatty acid sucrose ester, xylitol, mannitol crystal, natural menthol, citric acid, trisodium phosphate, sodium saccharin and steviosid; the above components are carried out the processes of crushing, mixing, uniformly stirring, packing and disinfecting to obtain the tea bag.
Owner:肖梅芬
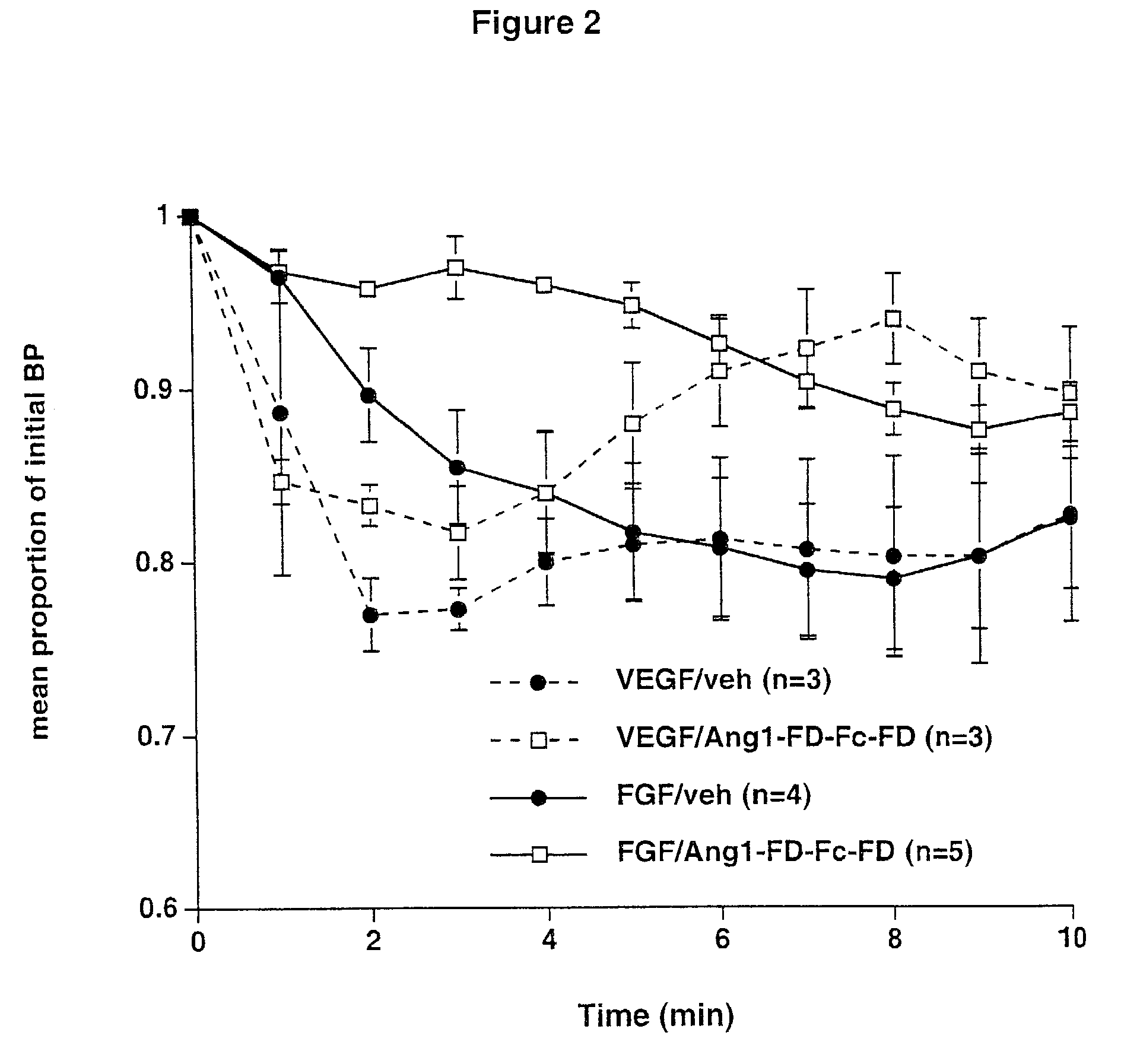
Angiopoietins and methods of treating hypertension
ActiveUS7052695B2Increase blood flowPeptide/protein ingredientsAntibody mimetics/scaffoldsArterial VasodilationHypotension shock
The invention generally relates to angiogenic factors and more particularly to the angiopoietin family of growth factors and to methods of using these growth factors to induce vasodilation and hypotension and reducing hypertension.
Owner:REGENERON PHARM INC
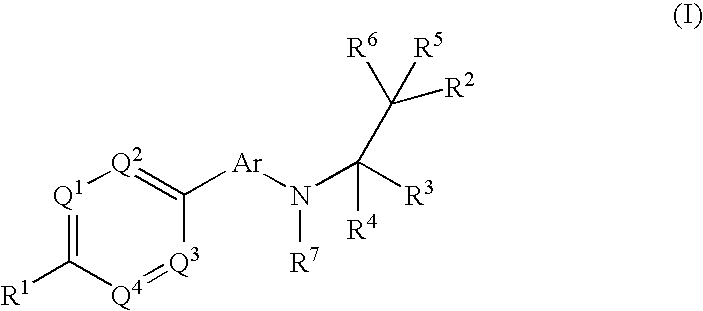
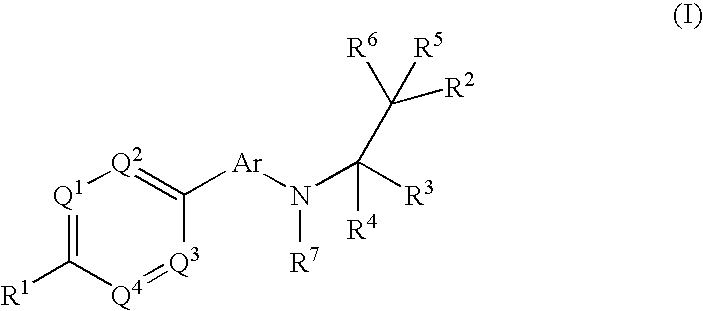
Phenyl or heteroaryl amino alkane derivatives as ip receptor antagonist
InactiveUS20060089371A1Excellent IP receptor antagonistic activitySuitable for productionBiocideOrganic active ingredientsVisceral painHeadaches
The present invention relates to phenyl or heteroaryl amino alkane derivatives of formula (I) in which the groups Q1-Q4, Ar, and R1-R7 are as defined in the specification and claims. These materials are useful as active ingredients of pharmaceutical preparations. The phenyl or heteroaryl amino alkanes of the present invention have IP receptor antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with IP receptor antagonistic activity. Such diseases include urological diseases or disorders as follows: bladder outlet obstruction, overactive bladder, urinary incontinence, detrusor hyper-reflexia, detrusor instability, reduced bladder capacity, frequency of micturition, urge incontinence, stress incontinence, bladder hyperreactivity, benighn prostatic hypertrophy (BPH), prostatitis, urinary frequency, nocturia, urinary urgency, pelvic hypersensitivity, urethritis, pelvic pain syndrome, prostatodynia, cystitis, or idiophatic bladder hypersensitivity. The compounds of the present invention are also useful for treatment of pain including, but not limited to inflammatory pain, neuropathic pain, acute pain, chronic pain, dental pain, premenstrual pain, visceral pain, headaches, and the like; hypotension; hemophilia and hemorrhage; and inflammation, since these diseases also are alleviated by treatment with an IP receptor antagonist. The application claims the compounds, pharmaceutical compositions containing them, and methods of treatment using them.
Owner:BAYER HEALTHCARE AG
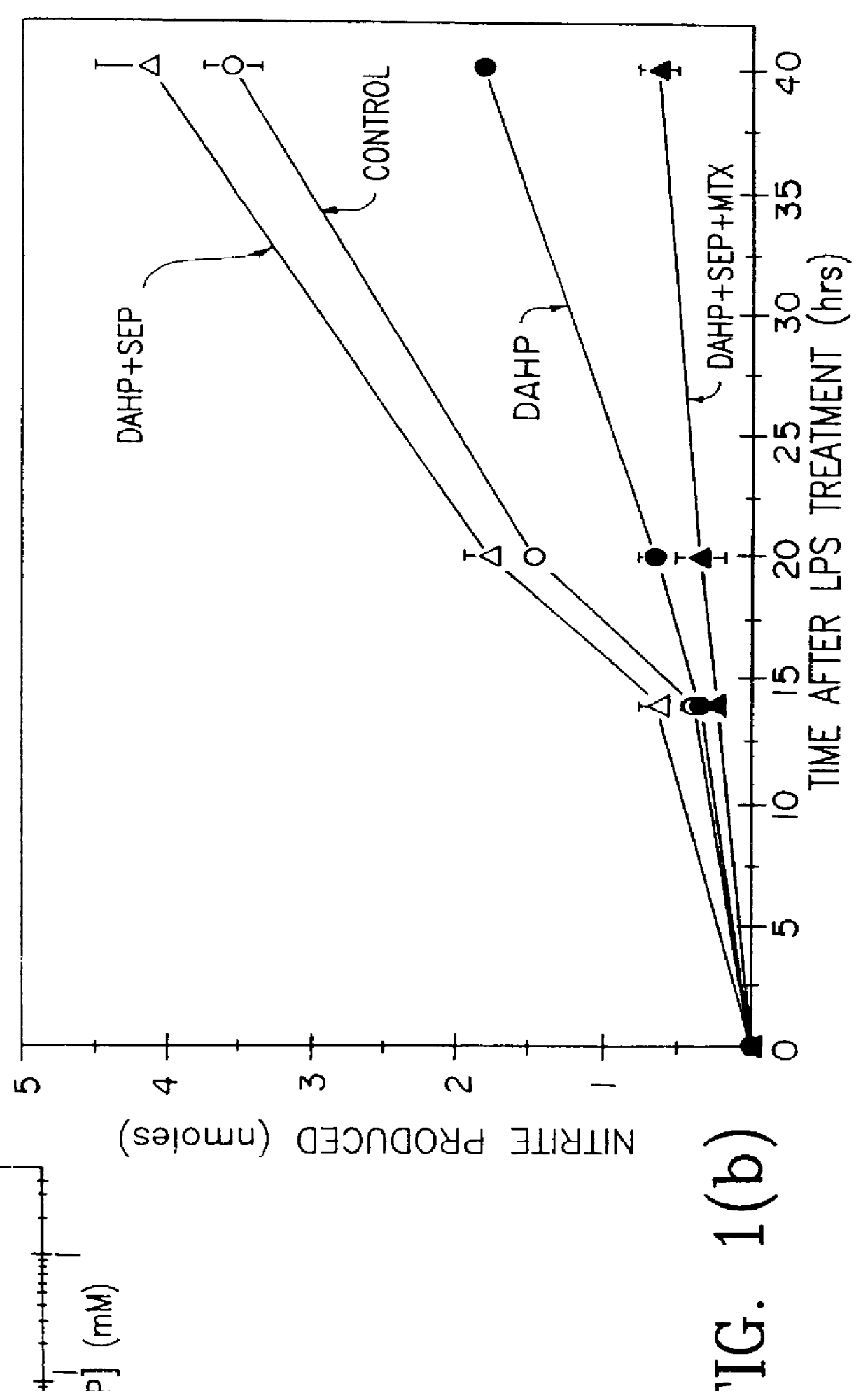
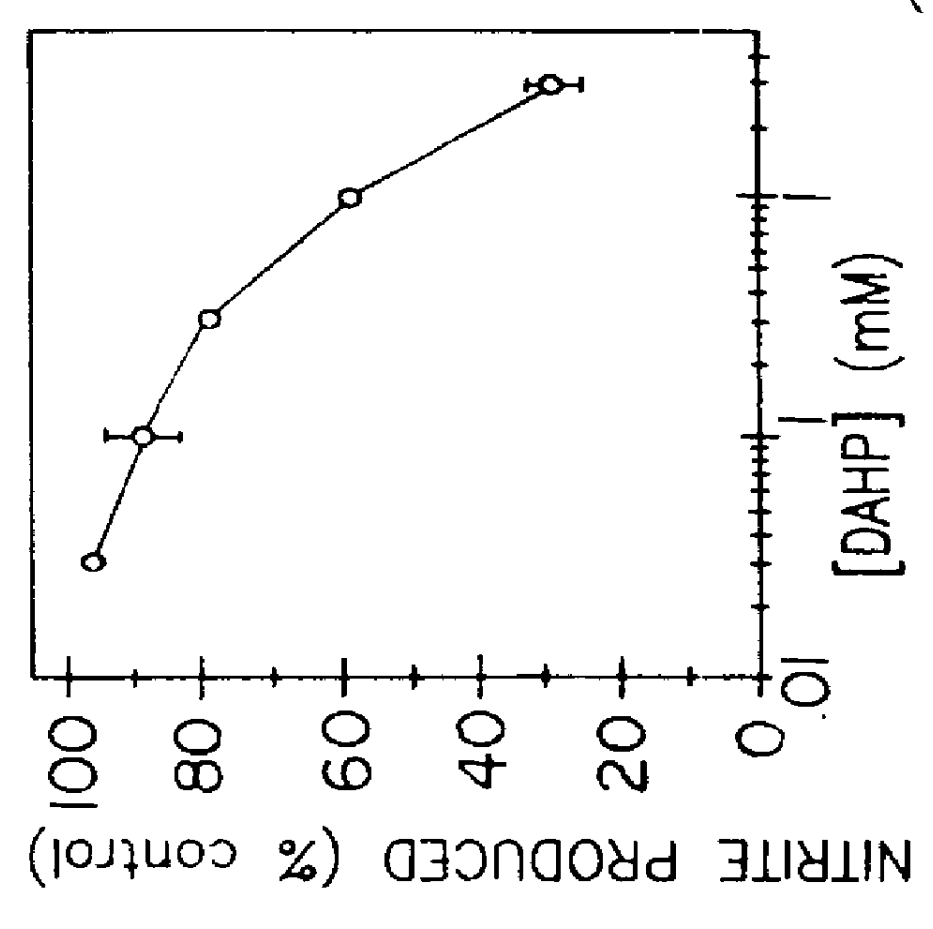
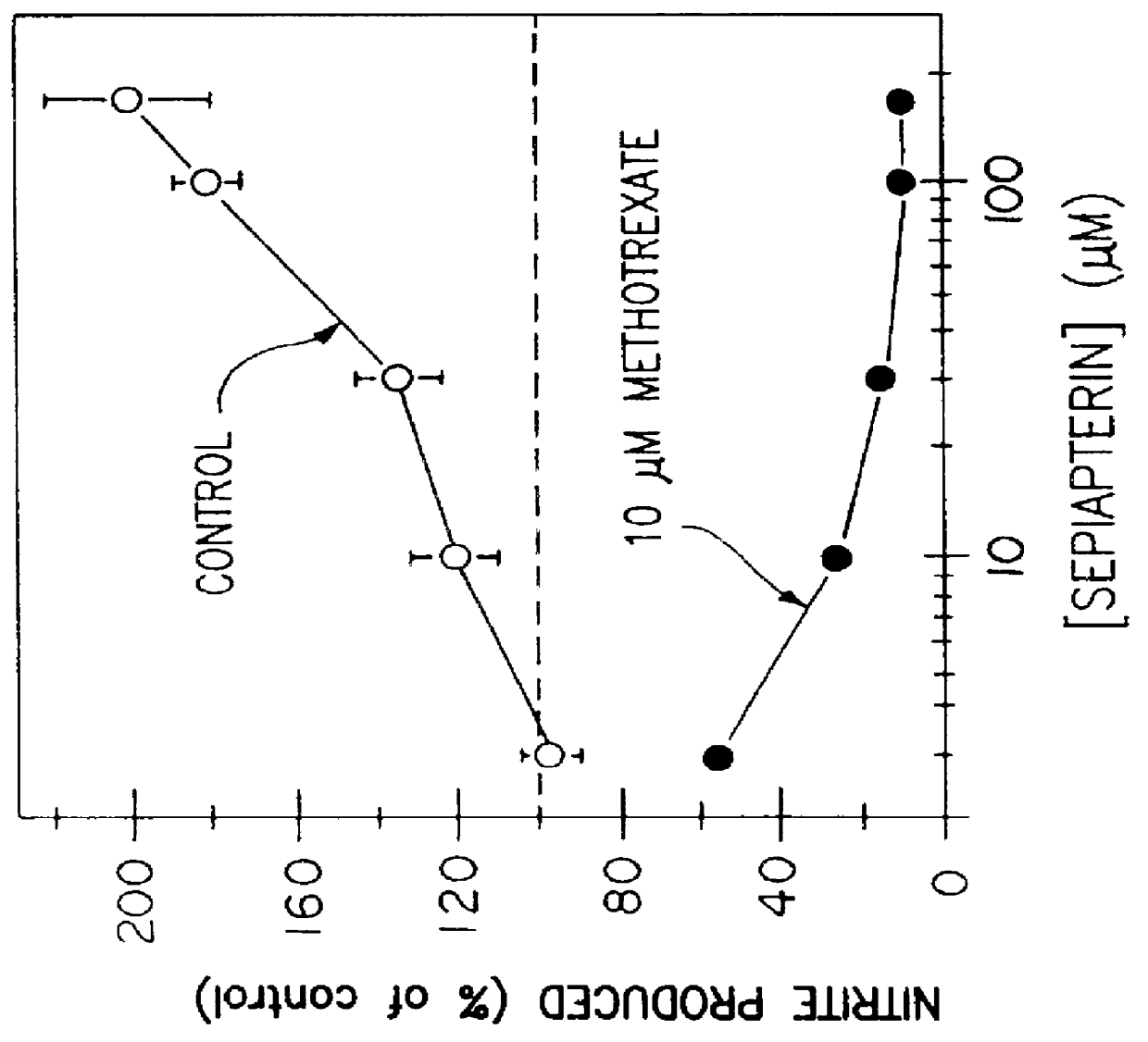
Blocking induction of tetrahydrobioterin to block induction of nitric oxide synthesis
InactiveUS6153615AReduce inhibitionRestore sensitivityBiocidePeptide/protein ingredientsSide effectTreatment effect
Guanosine triphosphate pathway tetrahydrobiopterin synthesis antagonist and / or pterin salvage pathway tetrahydrobiopterin synthesis antagonists are administered to inhibit nitric oxide synthesis from arginine in vascular cells in a subject in need of such inhibition (e.g., for prophylactic or curative effect for endotoxin- or cytokine-induced hypotension or for restoration of vascular contractile sensitivity to pressor agents in the treatment of such hypotension). The tetrahydrobiopterin synthesis antagonist may be administered with alpha 1-adrenergic agonist or with nitric oxide synthase inhibitor. The tetrahydrobiopterin synthesis antagonists are also administered to attenuate inflammation caused by induced nitric oxide production in immune cells. Unwanted counterproductive or side effects can be eliminated or ameliorated by administration additionally of levodopa with or without carbidopa and L-5-hydroxytryptophane.
Owner:CORNELL RES FOUNDATION INC
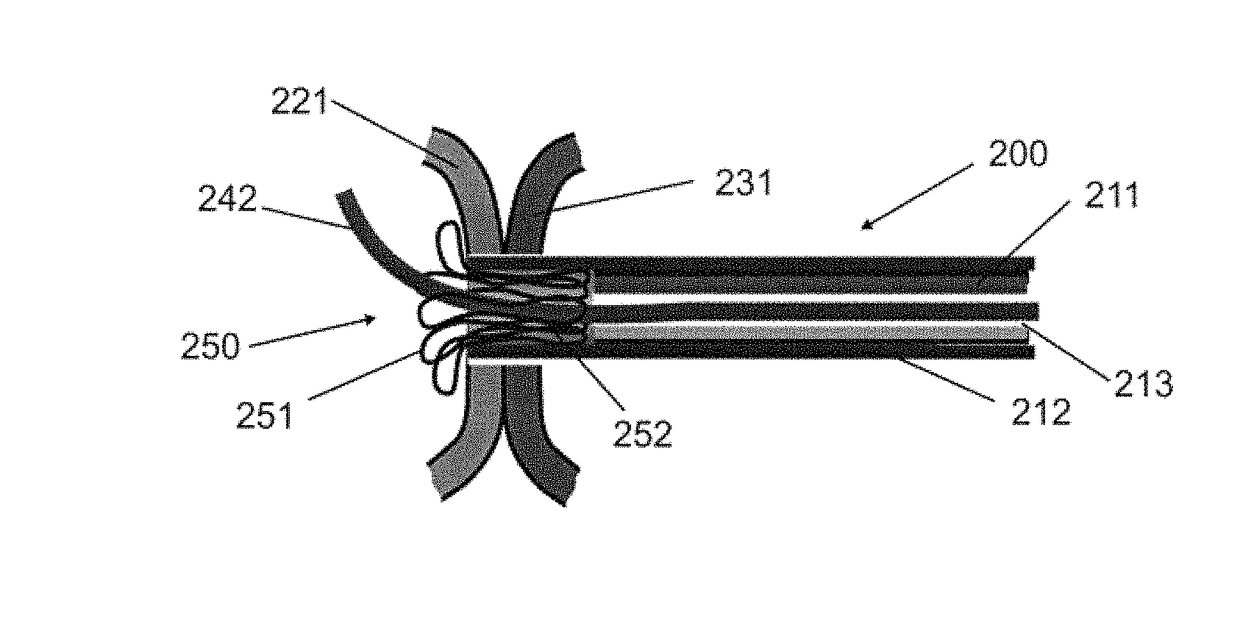
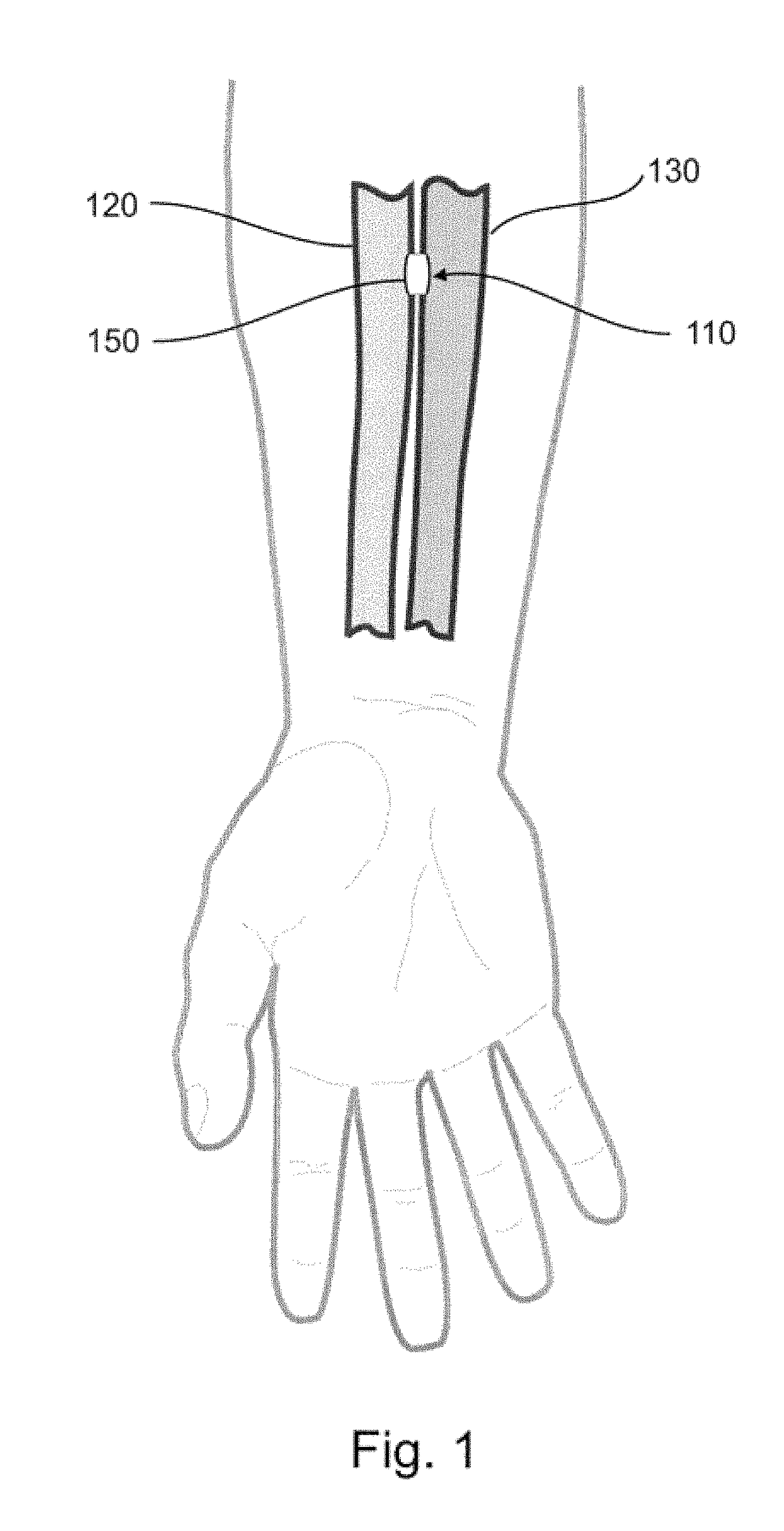
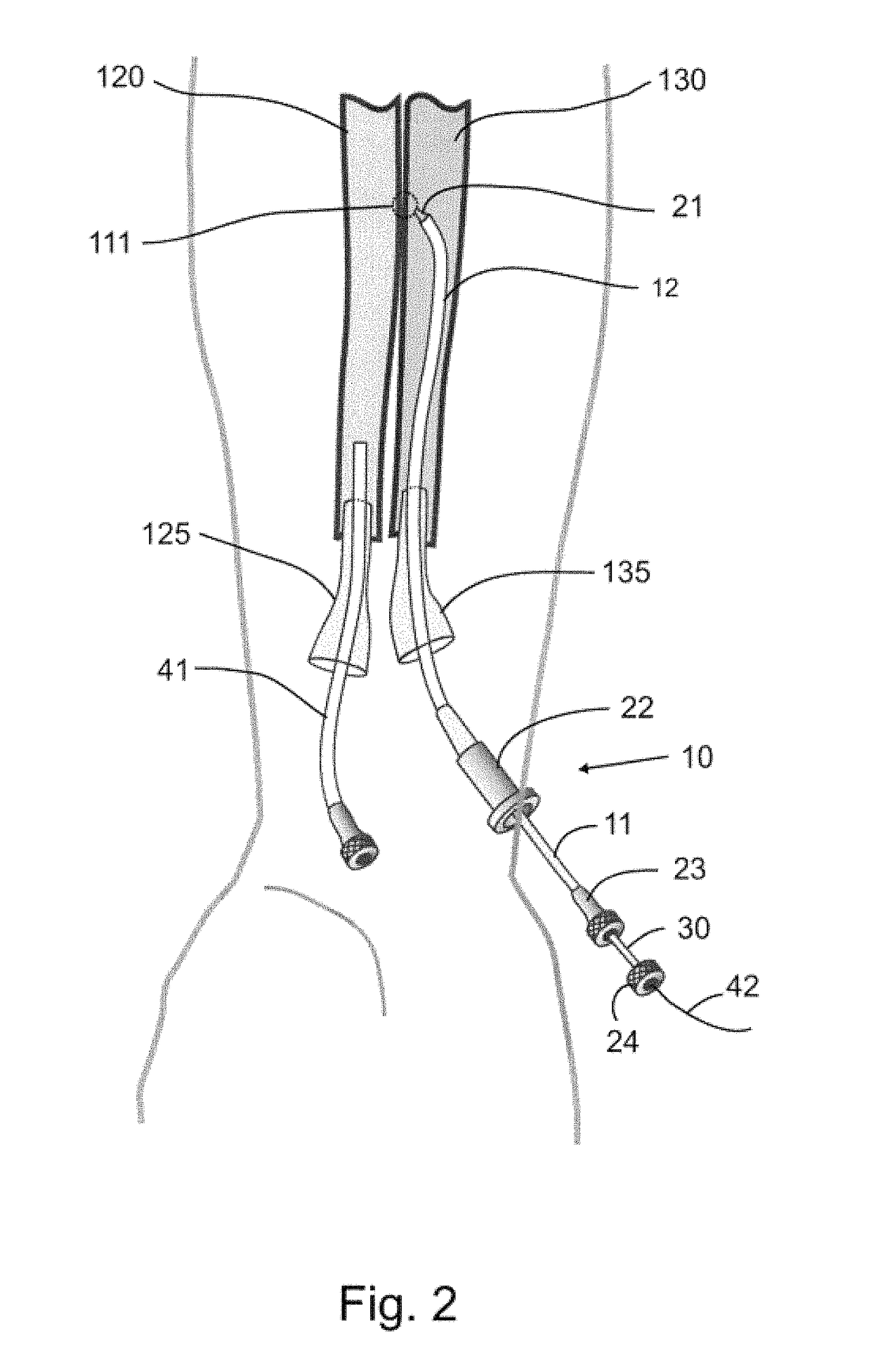
Devices, systems, and methods for peripheral arteriovenous fistula creation
ActiveUS9782533B2Good treatment effectReduce risk and adverse eventOther blood circulation devicesMedical devicesRESPIRATORY DISTRESS SYNDROME ADULTDisease
Owner:EDWARDS LIFESCIENCES CORP
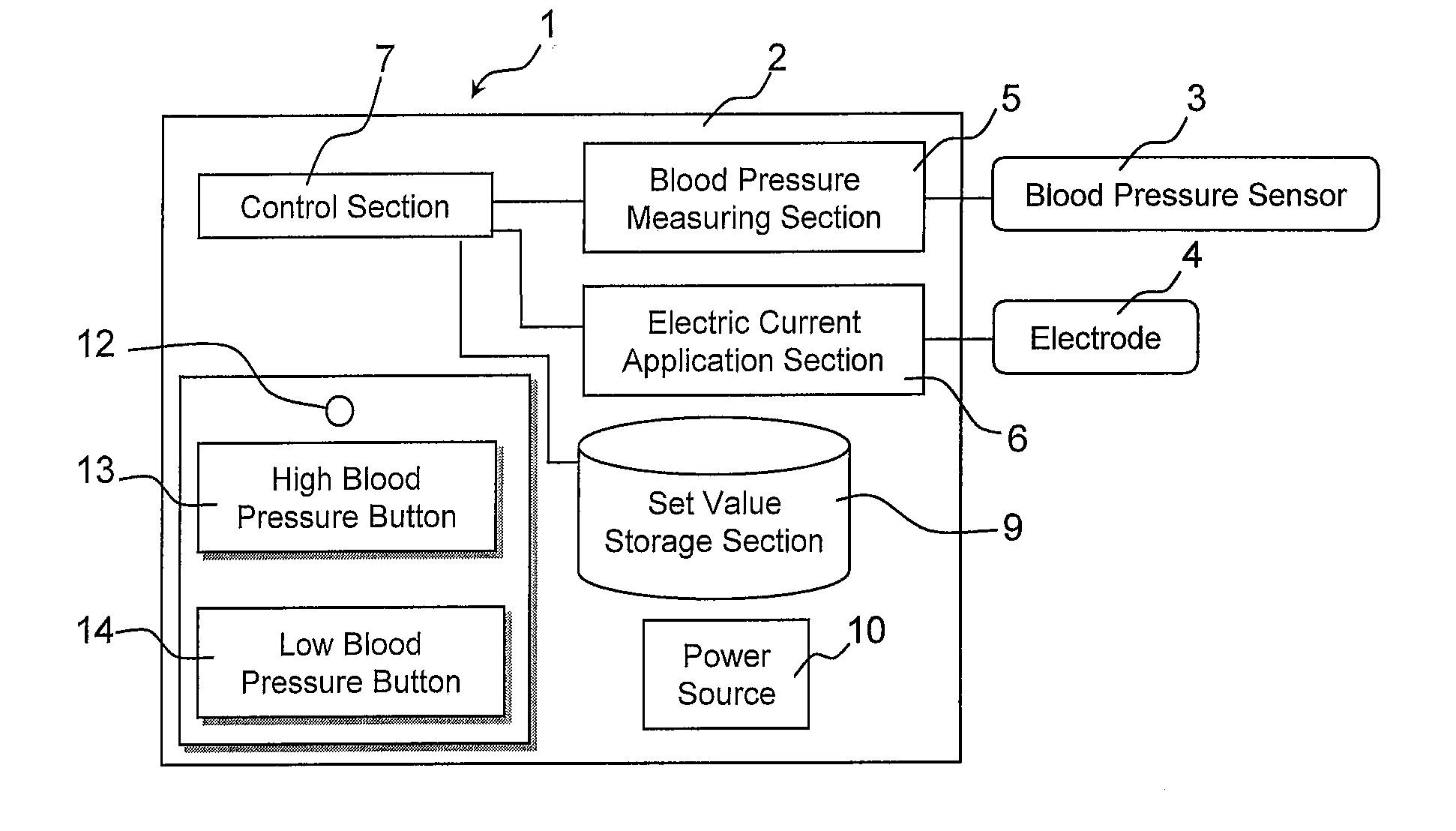
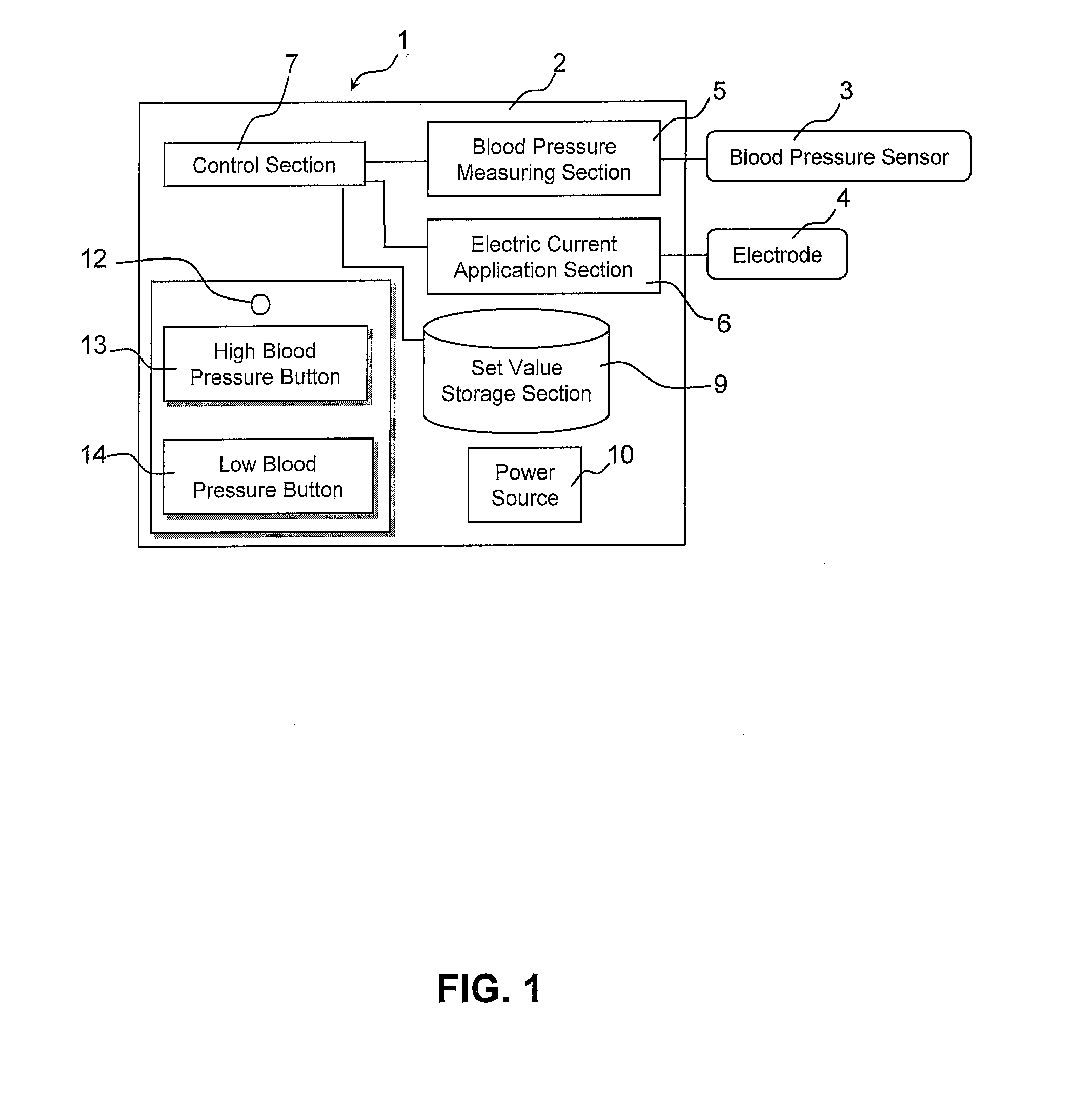
Blood pressure stabilization system using transdermal stimulation
InactiveUS20110202107A1Remarkable effectMinimal electrical powerElectrotherapyEvaluation of blood vesselsElectricityMedicine
The present invention relates to an electric stimulation apparatus for treating hypotension of patients with spinal cord injury and a method for treating hypotension. An electric stimulation apparatus of the present invention comprises: a blood pressure measuring means for continuously measuring a blood pressure of a subject; an electric current application means for intermittently applying an electric current to skin of the subject; and a control means for controlling the electric current application means so as to maintain the blood pressure at a predetermined target blood pressure value by activating the electric current application means when the subject blood pressure is equal to or less than the target blood pressure value.
Owner:KYUSHU UNIV
Benzimidazole derivatives
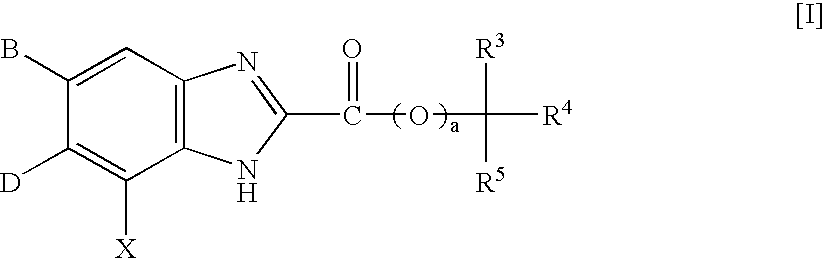
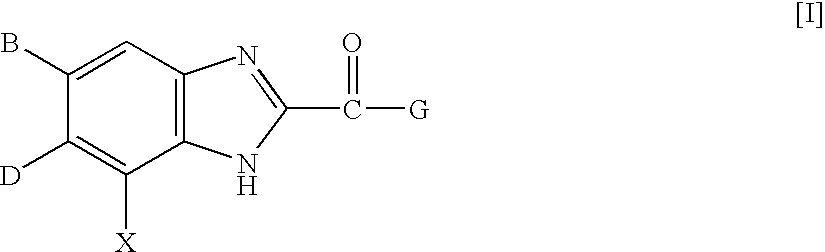
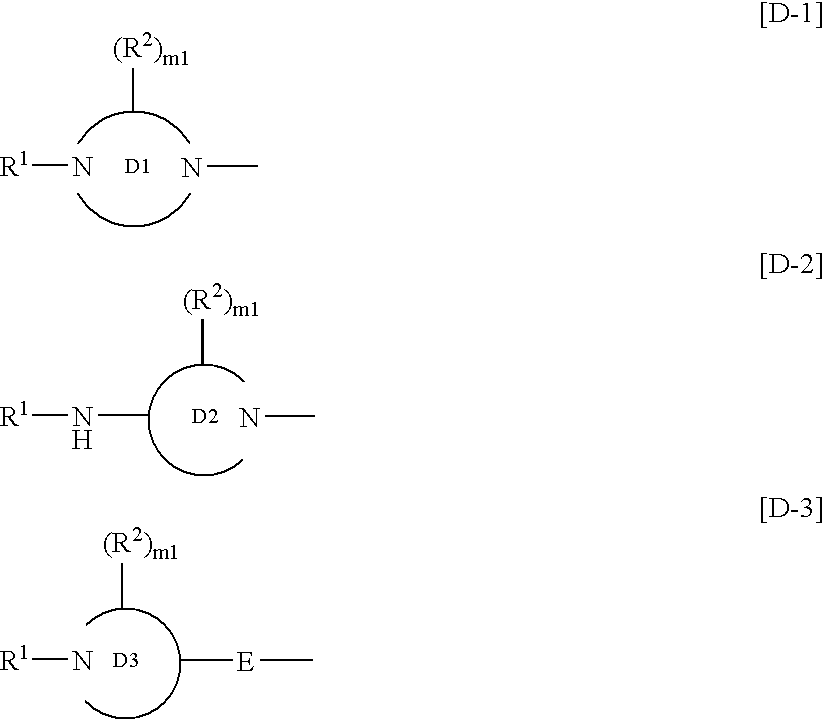
InactiveUS7125877B2High selectivityExcellent antagonismGroup 4/14 element organic compoundsBiocideBenzimidazole derivativeHypotension shock
This invention provides compounds which are represented by a general formula [I][in which X stands for hydrogen or halogen; B stands for halogen, cyano or optionally fluorine-substituted lower alkyl; D stands for a 3–10 membered aliphatic nitrogen-containing heterocyclic group; R3, R4 and R5 may be same or different, and each stands for hydrogen, lower alkyl optionally having substituent group(s) and the like; and a is 0 or 1]. These compounds exhibit high affinity to nociceptin receptors and whereby inhibit actions of nociceptin, and are useful as an analgesic, antiobestic, agent for ameliorating brain function, treating agents for Alzheimer's disease and dementia, and therapeutic agents for schizophrenia, neurodegenerative diseases, depression, diabetes insipidus, polyuria, hypotension and the like.
Owner:MSD KK
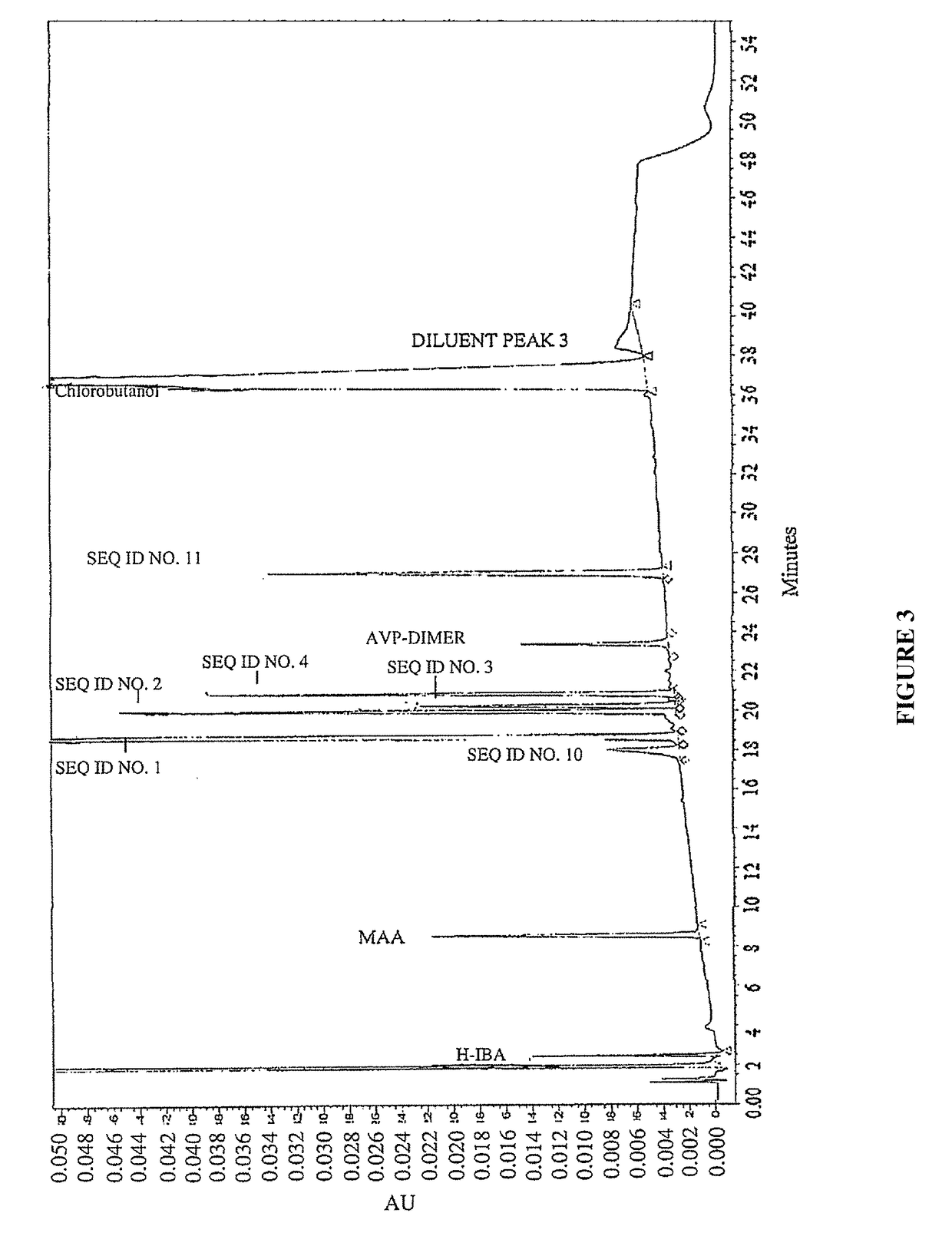
Vasopressin formulations for use in treatment of hypotension
ActiveUS9375478B1Reduce degradationHydroxy compound active ingredientsPeptide/protein ingredientsVasopressin preparationRoom temperature
Provided herein are peptide formulations comprising polymers as stabilizing agents. The peptide formulations can be more stable for prolonged periods of time at temperatures higher than room temperature when formulated with the polymers. The polymers used in the present invention can decrease the degradation of the constituent peptides of the peptide formulations.
Owner:PAR PHARMA
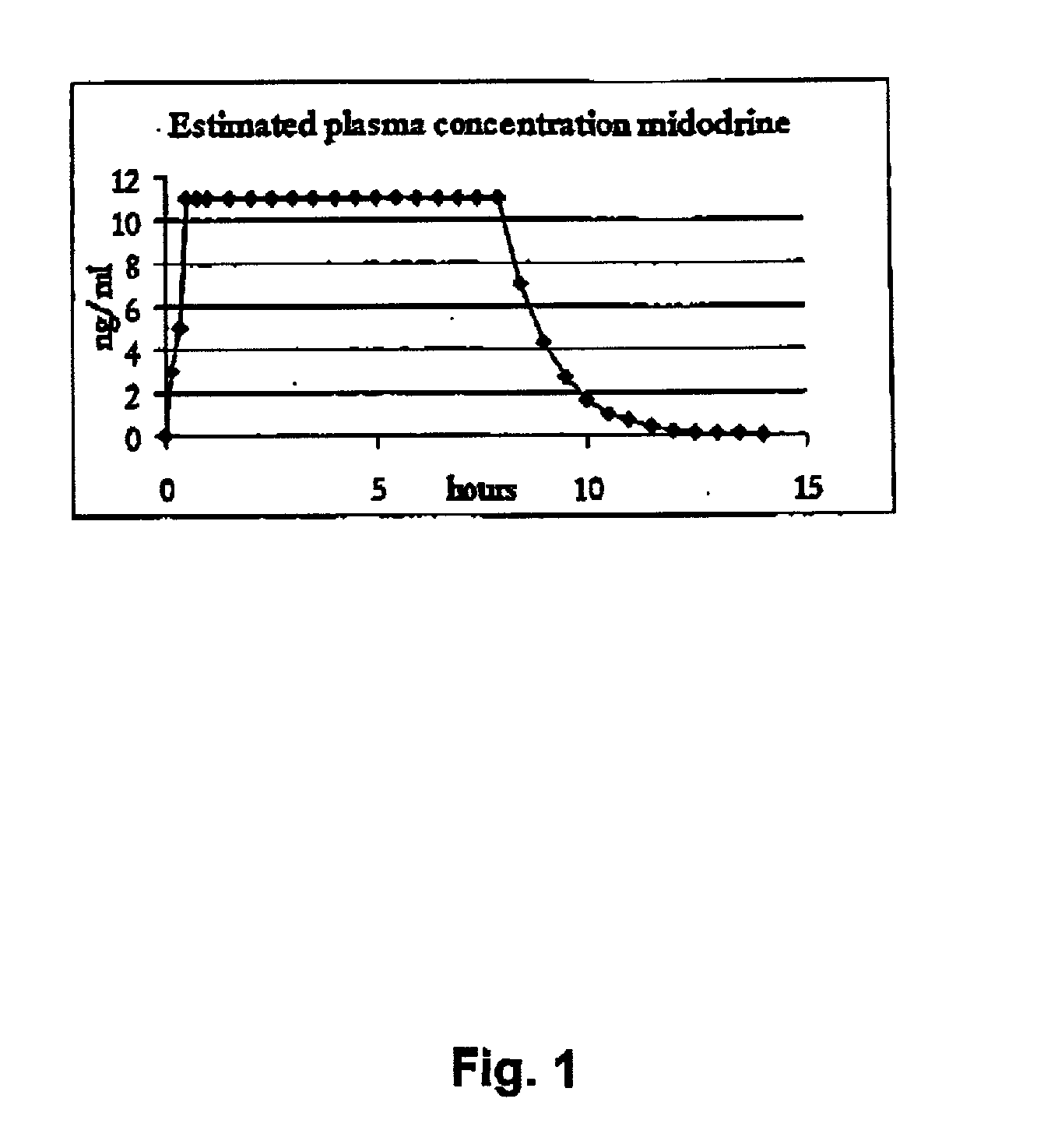
Pharmaceutical kit comprising midodrine as active drug substance
InactiveUS20020193445A1Fast curative effectRapid onsetBiocidePowder deliveryHigh concentrationSide effect
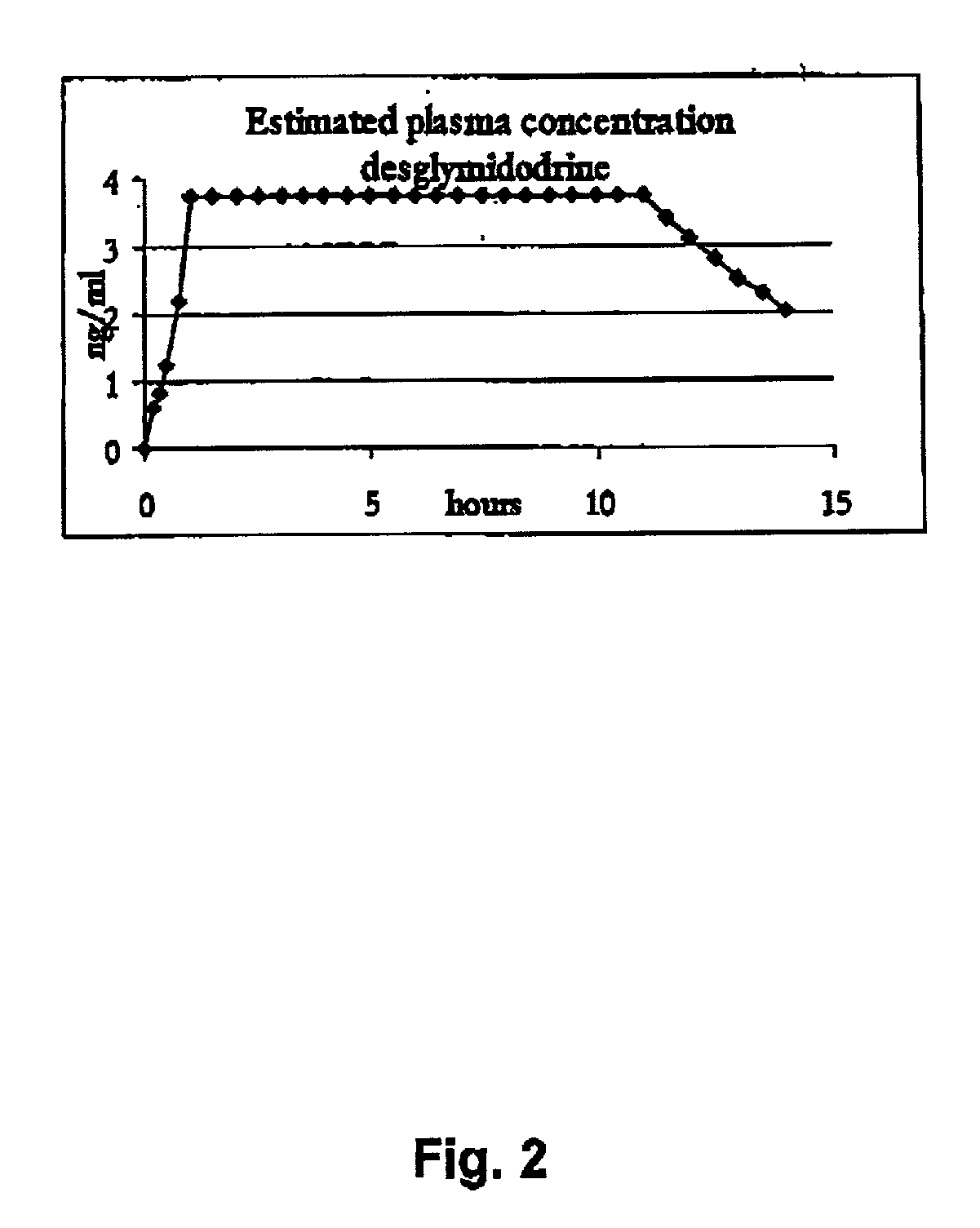
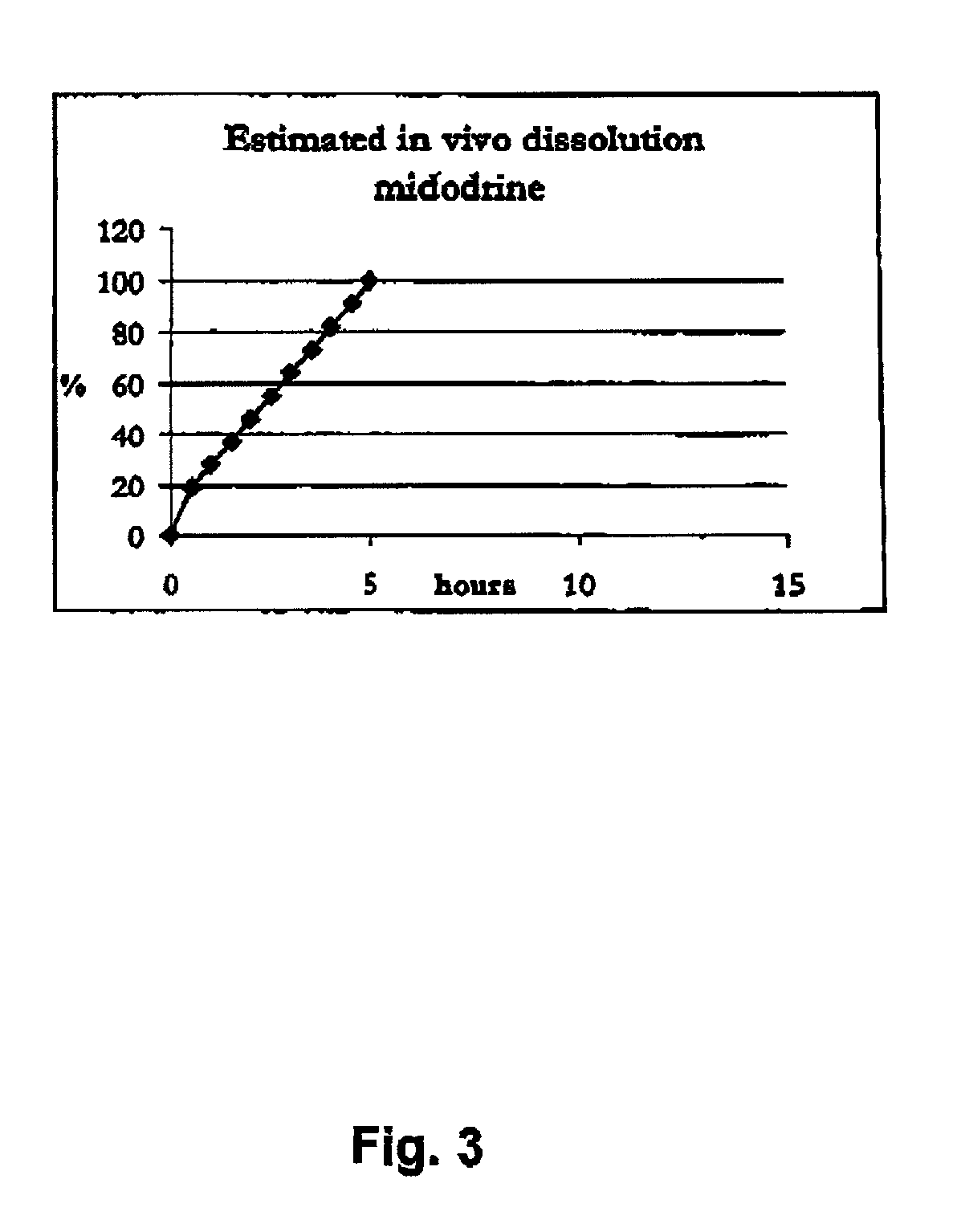
Novel phannaceutcal kit comprising a controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine and a relatively fast onset composition. The controlled release compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine. The controlled release compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps; i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desgtymidodrine have been released from the composition. One of the advantages of the invention is that the controlled release composition provides a base line plasma concentration, which during most of the day is therapeutically effective. When a higher concentration is needed, only a minor supply of active drug substance is necessary to obtain a very fast relief from symptoms. If the constant base line plasma concentration was absent, it would be necessary to use a relative higher fast onset dose to reach the high therapeutically effective level. The kit according to the present invention is a superior tool for obtaining an optimal treatment with a minimum of active drug substance. Also disclosed is a method for treating orthostaic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a kit according to the invention.
Owner:NYCOMED AUSTRIA
Imidazolyl derivatives
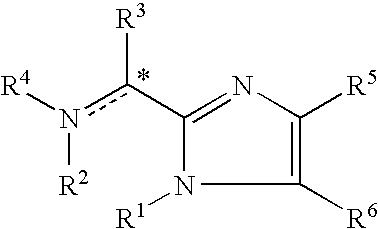
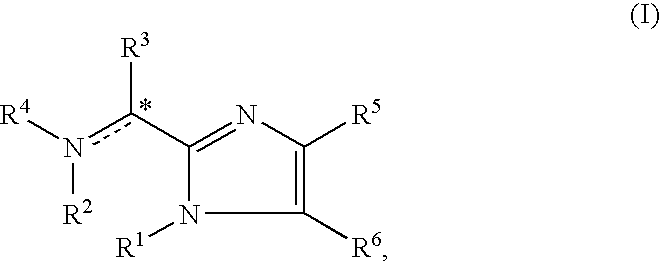
The present invention is directed to imidazolyl derivatives of the formula: where the substituents are defined in the specification, or a pharmaceutically acceptable salt thereof. The derivatives bind selectively to the somatostatin subtype receptors and elicit either an agonist or antagonist effect from the somatostatin subtype receptors. The derivatives are useful for treating a variety of diseases including acromegaly, restenosis, Crohn's disease, systemic sclerosis, external and internal pancreatic pseudocysts and ascites, VIPoma, nesidoblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, diarrhea, AIDS related diarrhea, chemotherapy related diarrhea, scleroderma, Irritable Bowel Syndrome, pancreatitis, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Cushing's Syndrome, gonadotropinoma, hyperparathyroidism, Graves' Disease, diabetic neuropathy, Paget's disease, polycystic ovary disease, cancer, cancer cachexia, hypotension, postprandial hypotension, panic attacks, GH secreting adenomas or TSH secreting adenomas.
Owner:IPSEN PHARMA SAS
Methods and compositions for the treatment and diagnosis of diseases characterized by vascular leak, hypotension, or a procoagulant state
InactiveUS20100221243A1Improve the situationReduce mortalityCompounds screening/testingOrganic active ingredientsHigh dosesIl-2 therapy
Disclosed herein are methods for treating a vascular leak disorder, hypotension, or a procoagulant state using angiopoietin-2 (Ang-2) antagonist compounds. Also disclosed are methods for treating a vascular leak disorder associated with high dose IL-2 therapy using angiopoietin-2 antagonist compounds. Methods for diagnosing and monitoring vascular leak disorders, hypotension, or a procoagulant state that include the measurement of Ang-2 polypeptide or nucleic acid levels are also disclosed. Methods for inducing a vascular leak using an Ang-2 agonist are also disclosed.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Controller for ultrafiltration blood circuit which prevents hypotension by monitoring osmotic pressure in blood
InactiveUS7399289B2Robust and inexpensiveSemi-permeable membranesSolvent extractionAutomatic controlUltrafiltration
A method and system for the extracorporeal treatment of blood to remove fluid from the fluid overloaded patient is disclosed that non-invasively measures osmotic pressure across a filter membrane of a blood filter. The filter is permeable to water and electrolytes, but not to blood protein. The osmotic pressure indicates the protein concentration in the blood. Osmotic pressure is used to detect when hypotension is about to occur in a patient, as a result of excessive blood volume reduction during treatment of the blood. Using the osmotic pressure measurement as a feedback signal, the rate of fluid extraction is automatically controlled to achieve the desired clinical outcome and avoid precipitating a hypotensive crisis in the patient.
Owner:GAMBRO LUNDIA AB
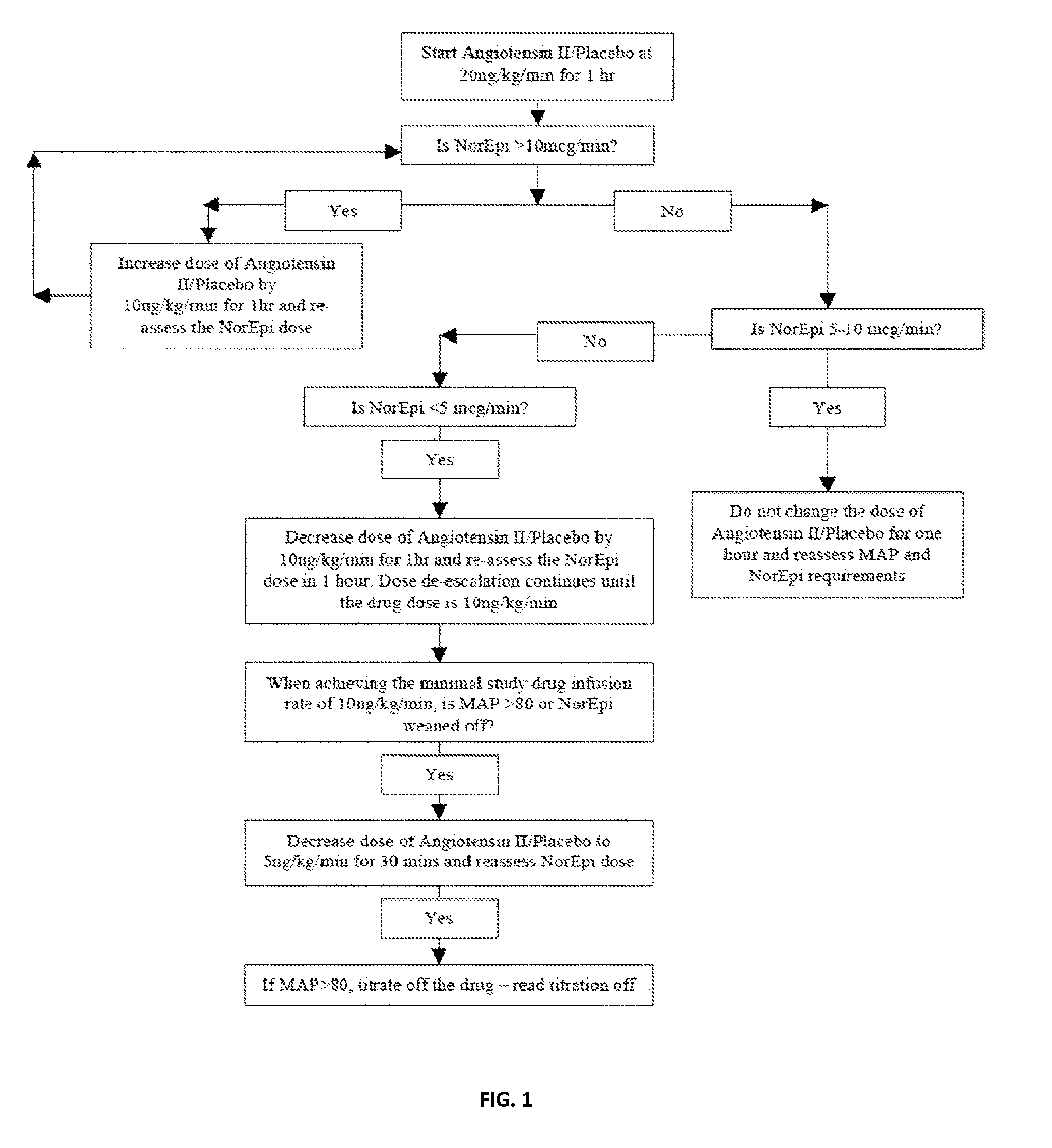
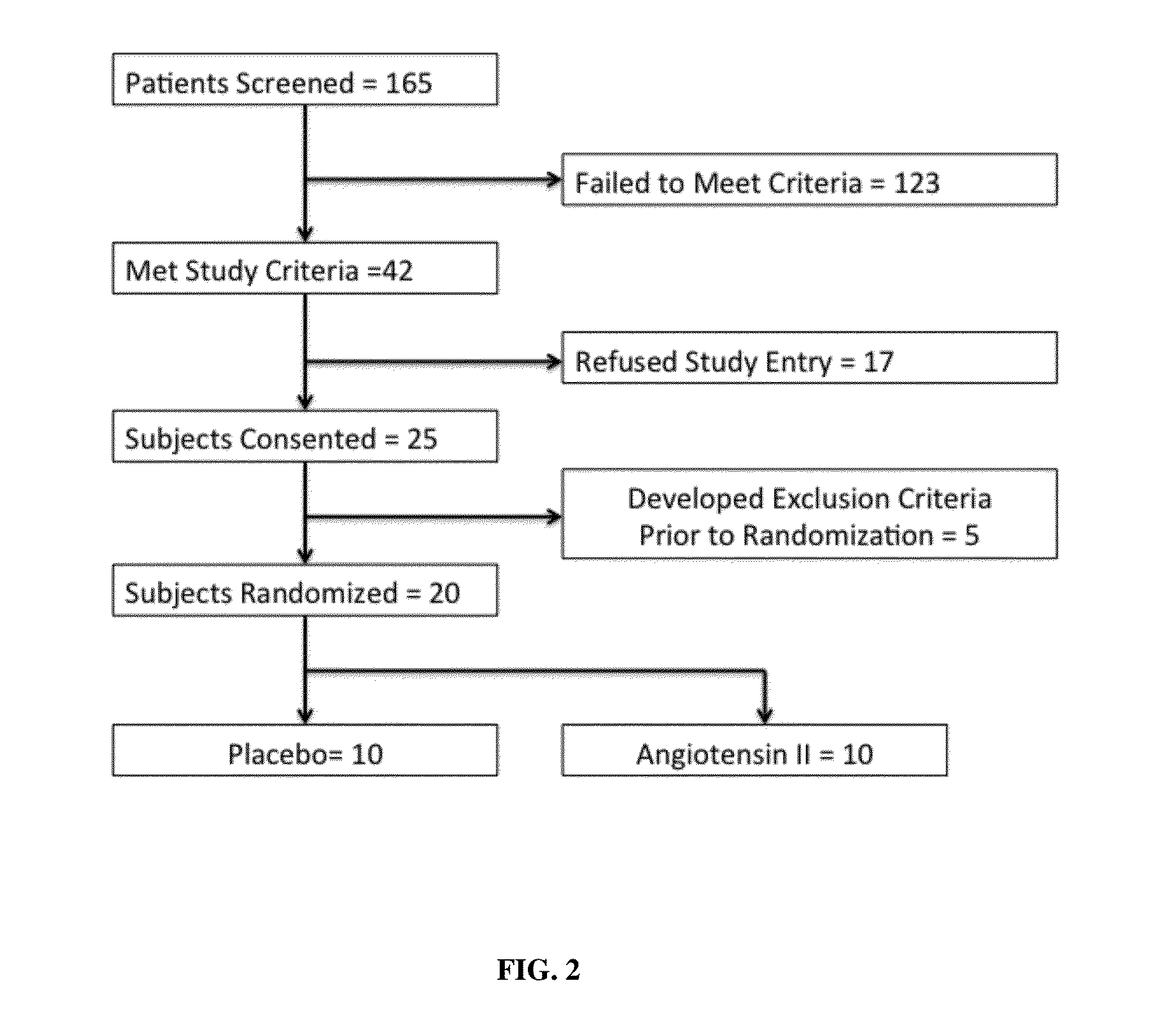
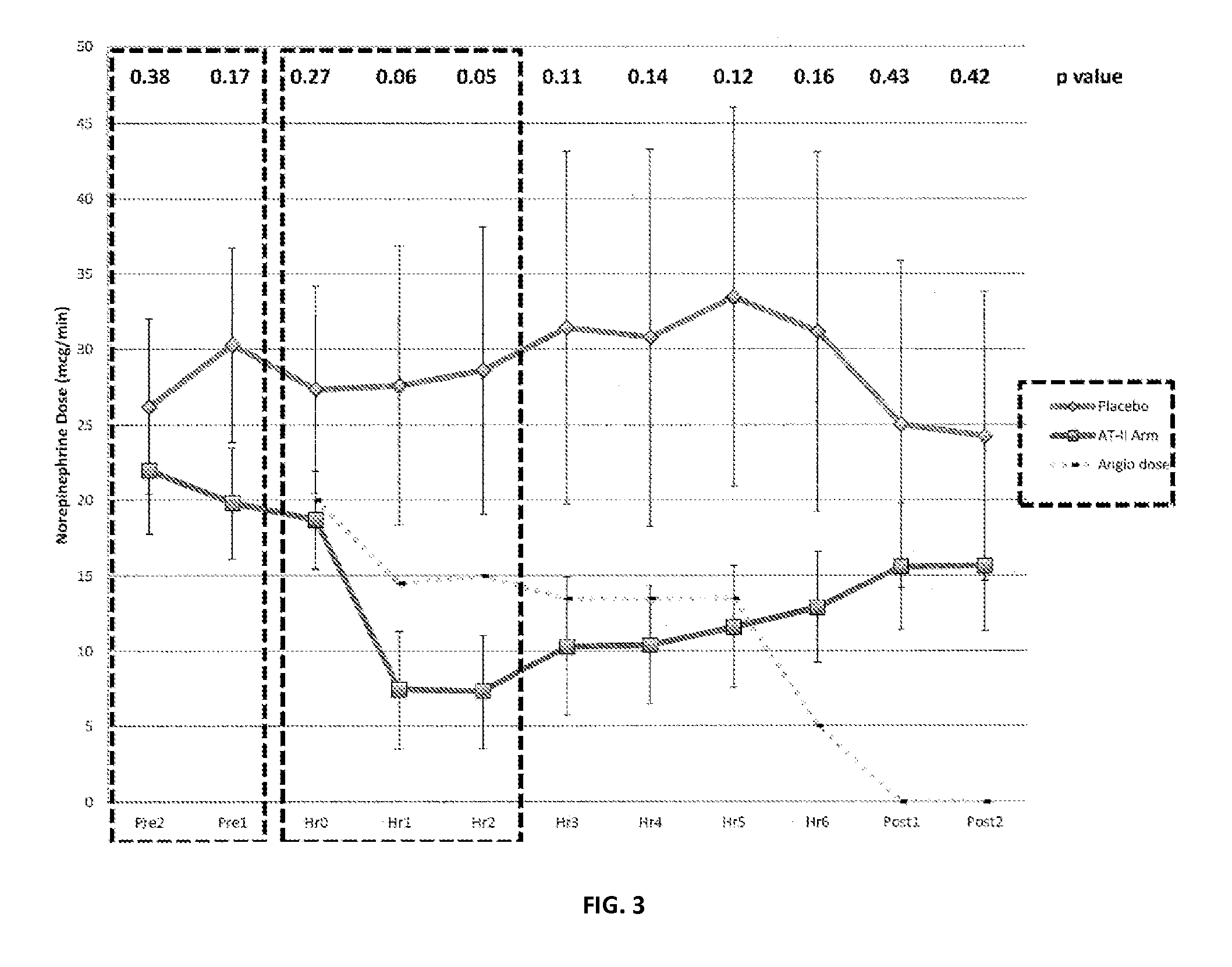
Angiotensin II alone or in combination for the treatment of hypotension
The present invention relates, inter alia, to a method comprising administering to a subject having high output shock and undergoing treatment with a catecholamine at a dose equivalent to at least about 0.2 mcg / kg / min of norepinephrine a dose of angiotensin II which is effective to raise the blood pressure of the subject to a mean arterial pressure (MAP) of about 65 mm Hg or above, and which is effective to reduce the dose of the catecholamine required to maintain a MAP of about 65 mm Hg to the equivalent of about 0.05-0.2 mcg / kg / min norepinephrine or less, or to the equivalent of about 0.05 mcg / kg / min norepinephrine or less.
Owner:THE GEORGE WASHINGTON UNIV A CONGRESSIONALLY CHARTERED NOT FOR PROFIT CORP
Method and drug composition for treating septic shock hypotension
Provided herein are methods and compositions for treating septic hypotension. More specifically, the methods may comprise, administering one alpha-2 agonist or other sympatholytic and at least one vasopressor.
Owner:QUINTIN LUC
Vasopressin formulations for use in treatment of hypotension
ActiveUS9744209B2Peptide/protein ingredientsComponent separationVasopressin preparationRoom temperature
Provided herein are peptide formulations comprising polymers as stabilizing agents. The peptide formulations can be more stable for prolonged periods of time at temperatures higher than room temperature when formulated with the polymers. The polymers used in the present invention can decrease the degradation of the constituent peptides of the peptide formulations.
Owner:PAR PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com